Nitrogen dioxide
| Nitrogen dioxide | |
|---|---|

| |
| |

| |
| Identifiers | |
| CAS number | [] |
| Properties | |
| Molecular formula | NO2 |
| Molar mass | 46.0055 |
| Appearance | brown gas |
| Density | 1443 kg/m³, liquid 3.4 kg/m³, gas at 294.25 K |
| Melting point |
-11.2°C (261.95 K) |
| Boiling point |
21.1°C (293.25 K) |
| Hazards | |
| EU classification | Highly toxic (T+) |
| NFPA 704 |
|
| R-phrases | R26, R34 |
| S-phrases | S1/2, S9, S26, S28,S36/37/39, S45 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Nitrogen dioxide is a chemical compound with the formula NO2. It is one of the several nitrogen oxides. At ordinary temperatures and atmospheric pressure, it is a reddish-brown gas with a characteristic sharp, biting odor. It is one of the most prominent air pollutants and a poison by inhalation. This gas is present in small quantities in smog and automobile exhaust fumes. However, chemists find it useful as a catalyst, nitrating agent, and oxidizing agent.
Preparation
Nitrogen dioxide (NO2) is prepared by simple reaction of nitric acid (HNO3) over copper metal. The reaction is the following:
- 4HNO3(aq) + Cu(s) → Cu(NO3)2(aq) + 2NO2(g) + 2H2O(L)
Safety and pollution considerations
Nitrogen dioxide is toxic by inhalation. Symptoms of poisoning (lung edema) tend to appear several hours after one has inhaled a low but potentially fatal dose. Also, low concentrations (4 ppm) will anesthetize the nose, thus creating a potential for overexposure.
Long-term exposure to NO2 at concentrations above 40–100 µg/m³ causes adverse health effects[1].
Nitrogen dioxide is formed in most combustion processes using air as the oxidant. At elevated temperatures nitrogen combines with oxygen to form nitrogen dioxide:
- 2O2 + N2 → 2 NO2
The most important sources of NO2 are internal combustion engines [2], thermal power stations and, to a lesser extent, pulp mills.[3]
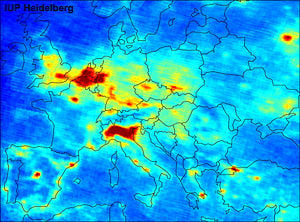
The map shown below, depicting results of satellite measurements over Europe, illustrates nitrogen dioxide as large scale pollutant, with rural background ground level concentrations in some areas around 30 µg/m³, not far below unhealthful levels. Nitrogen dioxide plays a role in atmospheric chemistry, including the formation of tropospheric ozone. A recent study by researchers at the University of California, San Diego, suggests a link between NO2 levels and Sudden Infant Death Syndrome. [4]
Oxides of nitrogen
- Nitrous oxide or N2O, "laughing gas," a linear molecule, isoelectronic with CO2 but with a nonsymmetric arrangement of atoms (NNO)
- Nitric oxide or NO, a problematic pollutant that is short lived because it converts to NO2 in the presence of free oxygen.
- NOx = all of the above in unspecified proportions but tending toward NO2.
More esoteric nitrogen oxides include N2O5 and the blue species N2O3.
Oxidized (cationic) and reduced (anionic) derivatives of many of these oxides exist: nitrite (NO2−), nitrate (NO3−), nitronium or NO2+, and nitrosonium or NO+. NO2 is intermediate between nitrite and nitronium:
- NO2+ + e− → NO2
- NO2 + e− → NO2−
See also
Notes
- ↑ Health Aspects of Air Pollution with Particulate Matter,Ozone and Nitrogen Dioxide. Retrieved May 25, 2008..
- ↑ Son, Busoon and Wonho Yang, Patrick Breysse, Taewoong Chung and Youngshin Lee (March 2004). Estimation of occupational and nonoccupational nitrogen dioxide exposure for Korean taxi drivers using a microenvironmental model. Environmental Research 94 (3): 291-296.
- ↑ Air emissions. Botnia. Retrieved May 25, 2008.
- ↑ Sids Linked to Nitrogen Dioxide Pollution. Retrieved May 25, 2008.
ReferencesISBN links support NWE through referral fees
- Chang, Raymond. 2006. Chemistry, 9th ed. New York: McGraw-Hill Science/Engineering/Math. ISBN 0073221031.
- Cotton, F. Albert, Geoffrey Wilkinson, Carlos A. Murillo, and Manfred Bochmann. 1999. Advanced Inorganic Chemistry 6th ed. New York: Wiley. ISBN 0471199575.
- Sloss, Leslie L. 1992. Nitrogen Oxides Control Technology Fact Book. Park Ridge, NJ: Noyes Data Corp. ISBN 0815512945.
External links
All links retrieved November 15, 2022.
- NIOSH Pocket Guide to Chemical Hazards
- Health Aspects of Air Pollution (2003) – WHO-Europe reports: (PDF)
- Nitrogen Dioxide Air Pollution
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.