Phosphoric acid
| Phosphoric acid | |
|---|---|
| |
| General | |
| Other names | Orthophosphoric acid |
| Molecular formula | H3PO4 |
| SMILES | OP(O)(O)=O |
| Molar mass | 98.0 g/mol |
| Appearance | white solid or colourless, viscous liquid (>42°C) |
| CAS number | 7664-38-2 |
| Properties | |
| Density and phase | 1.685 g/ml, liquid |
| Solubility in water | miscible |
| Melting point | 42.35 °C, 107.6°F, 567.27°R |
| Boiling point | 158 °C, 415.4°F, 875.1°R decomp. |
| Acidity (pKa) | 2.12, 7.21, 12.67 |
| Viscosity | 85% aqueous solution ? cP at ? °C |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Corrosive (C) |
| NFPA 704 | |
| R-phrases | R34 |
| S-phrases | S1/2, S26, S45 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | Nitric acid Arsenic acid |
| Other cations | Ammonium phosphate Trisodium phosphate |
| Related Phosphorus acids | Hypophosphorous acid Phosphorous acid Pyrophosphoric acid Tripolyphosphoric acid Hypophosphoric acid Perphosphoric acid Permonophosphoric acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Phosphoric acid, also known as orthophosphoric acid or phosphoric(V) acid, is a mineral (inorganic) acid having the chemical formula H3PO4. Alternatively, orthophosphoric acid molecules can combine with themselves to form a variety of compounds referred to as phosphoric acids in a more general way. The term "phosphoric acid" can also refer to a chemical or reagent consisting of phosphoric acids, usually mostly orthophosphoric acid.
Preparation of orthophosphoric acid
There are two distinct kinds of orthophosphoric acid, based on the method of preparation. They are known as thermal phosphoric acid and wet phosphoric acid.
Thermal phosphoric acid: This very pure phosphoric acid is obtained by burning elemental phosphorus to produce phosphorus pentoxide and dissolving the product in dilute phosphoric acid. This is the cleanest way of producing phosphoric acid, since most impurities present in the rock have been removed when extracting phosphorus from the rock in a furnace. The end result is food grade, thermal phosphoric acid; however, for critical applications additional processing to remove arsenic compounds may be needed.
Wet phosphoric acid: Green phosphoric acid is prepared by adding sulfuric acid to calcium phosphate rock, or slurry. The reaction for calcium phosphate slurry is: 3H2SO4(aq) + Ca3(PO4)2(aq) + 6H2O(l) ↔ 2H3PO4(aq) + 3CaSO4(aq)+ 6H2O(l)
Through modern filtering techniques the wet process acid can be cleaned up significantly but still isn't as pure as thermal phosphoric acid; as it may contain other acidic species such as hydrofluoric acid.
Orthophosphoric acid chemistry
Pure anhydrous phosphoric acid is a white solid that melts at 42.35 °C to form a colorless, viscous liquid. Pure 75-85 percent aqueous solutions (the most common) are clear, colorless, odorless, non-volatile, rather viscous, syrupy liquids, but still pourable.
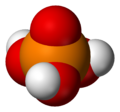
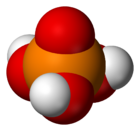
Most people and even chemists simply refer to orthophosphoric acid as "phosphoric acid," which is the IUPAC name for this compound. The prefix ortho- usually is used when one wants to distinguish it from other phosphoric acids called polyphosphoric acids. Orthophosphoric acid is a non-toxic, inorganic, rather weak triprotic acid which, when pure, is a solid at room temperature and pressure. The chemical structure of orthophosphoric acid is shown in the data table.
Orthophosphoric acid is a very polar molecule, therefore it is highly soluble in water. The oxidation state of phosphorus (P) in ortho- and other phosphoric acids is +5; the oxidation state of all the oxygens (O) is -2 and all the hydrogens (H) is +1. Triprotic means that an orthophosphoric acid molecule can dissociate up to three times, giving up an H+ each time, which typically combines with a water molecule, H2O, as shown in these reactions:
- H3PO4(s) + H2O(l) ⇌ H3O+(aq) + H2PO4–(aq) Ka1= 7.5×10−3
- H2PO4–(aq)+ H2O(l) ⇌ H3O+(aq) + HPO42–(aq) Ka2= 6.2×10−8
- HPO42–(aq)+ H2O(l) ⇌ H3O+(aq) + PO43–(aq) Ka3= 2.14×10−13
The anion after the first dissociation, H2PO4–, is the dihydrogen phosphate anion. The anion after the second dissociation, HPO42–, is the hydrogen phosphate anion. The anion after the third dissociation, PO43–, is the phosphate or orthophosphate anion. For each of the dissociation reactions shown above, there is a separate acid dissociation constant, called Ka1, Ka2, and Ka3 given at 25°C. Associated with these three dissociation constants are corresponding pKa1=2.12, pKa2=7.21, and pKa3=12.67 values at 25°C. Even though all three hydrogen (H) atoms are equivalent on an orthophosphoric acid molecule, the successive Ka values differ since it is energetically less favorable to lose another H+ if one (or more) has already been lost and the molecule/ion is more negatively charged.
Because the triprotic dissociation of orthophosphoric acid, the fact that its conjugate bases (the phosphates mentioned above) cover a wide pH range, and because phosphoric acid/phosphate solutions are generally non-toxic, mixtures of these types of phosphates are often used as buffering agents or to make buffer solutions, where the desired pH depends on the proportions of the phosphates in the mixtures. Similarly, the non-toxic, anion salts of triprotic organic citric acid are also often used to make buffers. Phosphates are found pervasively in biology, especially in the compounds derived from phosphorylated sugars, such as DNA and RNA and adenosine triphosphate (ATP). There is a separate article on phosphate as an anion or its salts.
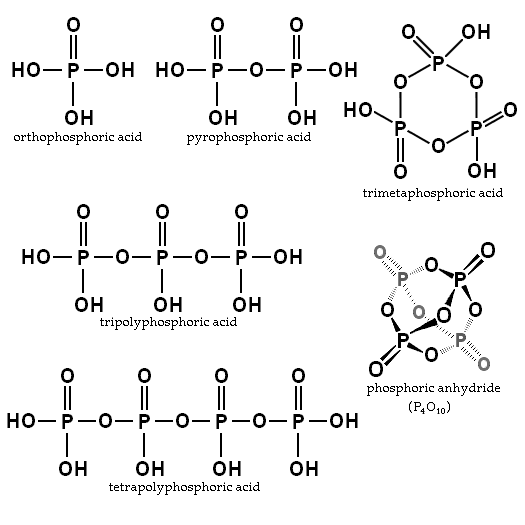
Upon heating orthophosphoric acid, condensation of the phosphoric units can be induced by driving off the water formed from condensation. When one molecule of water has been removed for each two molecules of phosphoric acid, the result is pyrophosphoric acid (H4P2O7). When an average of one molecule of water per phosphoric unit has been driven off, the resulting substance is a glassy solid having an empirical formula of HPO3 and is called metaphosphoric acid.[1] Metaphosphoric acid is a singly anhydrous version of orthophosphoic acid and is sometimes used as a water- or moisture-absorbing reagent. Further dehydrating is very difficult and can only be accomplished by means of an extremely strong desiccant (and not by heating alone). It produces phosphoric anhydride which has an empirical formula P2O5, although an actual molecule has a chemical formula of P4O10. Phosphoric anhydride is a solid which is very strongly moisture-absorbing and is used as a desiccant.
Phosphoric acid is very commonly used as an aqueous solution of 85 percent phosphoric acid or H3PO4. Because it is a concentrated acid, an 85 percent solution can be corrosive, although not toxic when diluted. Because of the high percentage of phosphoric acid in this reagent, at least some of the orthophosphoric acid is condensed into polyphosphoric acids in a temperature-dependent equilibrium, but for the sake of labeling and simplicity, the 85 percent represents H3PO4 as if it were all orthophosphoric acid. Other percentages are possible too, even above 100 percent, where the phosphoric acids and water would be in an unspecified equilibrium, but the overall elemental mole content would be considered specified. When aqueous solutions of phosphoric acid and/or phosphate are dilute, they are in or will reach an equilibrium after a while where practically all the phosphoric/phosphate units are in the ortho- form.
Uses of orthophosphoric acid
Rust removal
Phosphoric acid may be used by direct application to rusted iron, steel tools or surfaces to convert iron(III) oxide (rust) to a water soluble phosphate compound. It is usually available as a greenish liquid, suitable for dipping (acid bath), but is more generally used as a component in a gel, commonly called Naval jelly. As a thick gel, it may be applied to sloping, vertical, or even overhead surfaces. Care must be taken to avoid acid burns of the skin and especially the eyes, but the residue is easily diluted with water. When sufficiently diluted it can even be nutritious to plant life, containing the essential nutrients phosphorus and iron. It is sometimes sold under other names, such as "rust remover" or "rust killer." It should not be directly introduced into surface water such as creeks or into drains, however. After treatment, the reddish-brown iron oxide will be converted to a black iron phosphate compound coating that may be scrubbed off. Multiple applications of phosphoric acid may be required to remove all rust. The resultant black compound can provide further corrosion resistance (such protection is somewhat provided by the superficially similar Parkerizing and blued electrochemical conversion coating processes.) After application and removal of rust using phosphoric acid compounds, the metal should be oiled (if to be used bare, as in a tool) or appropriately painted, most durably by using a multiple coat process of primer, intermediate, and finish coats.
Processed food use
It is also used to acidify foods and beverages such as various colas, but not without controversy as to its health effects. It provides a tangy taste, and being a mass-produced chemical, is available cheaply and in large quantities. The low cost and bulk availability is unlike more expensive natural seasonings that give comparable flavors, such as ginger for tangyness, or citric acid for sourness, obtainable from lemons and limes. (However most citric acid in the food industry is not extracted from citrus fruit, but fermented by Aspergillus niger mold from scrap molasses, waste starch hydrolysates and phosphoric acid.) It is labeled as E number E338.
Medical use
Phosphoric acid is used in dentistry and orthodontics as an etching solution, to clean and roughen the surfaces of teeth where dental appliances or fillings will be placed. Phosphoric acid is also an ingredient in over the counter anti-nausea medications which also contain high levels of sugar (glucose and fructose). It should not be used by diabetics without consultation with a doctor. Phosphoric acid is also used as a catalyst in the synthesis of aspirin because it provides a larger number of hydrogen ions with less contamination when compared to hydrochloric acid and sulfuric acid.[2]
Preparation of hydrogen halides
Phosphoric acid reacts with halides to form the corresponding hydrogen halide gas
(steamy fumes are observed on warming the reaction mixture).
This is a common practice for the laboratory preparation of hydrogen halides.
3NaCl(s)+H3PO4(l)->NaH2PO4(s)+HCl(g)
3NaBr(s)+H3PO4(l)->NaH2PO4(s)+HBr(g)
3NaI (s)+H3PO4(l)->NaH2PO4(s)+HI (g)
Other applications
- Orthophosphoric acid is used as the electrolyte in phosphoric-acid fuel cells.
- It is used as an external standard for phosphorus-31 NMR.
- It is used as a cleaner by construction trades to remove mineral deposits, cementitious smears, and hard water stains. It is also used as an ingredient in some household cleaners aimed at similar cleaning tasks.
- Hot phosphoric acid is used in microfabrication to etch silicon nitride (Si3N4). It is highly selective in etching Si3N4 instead of SiO2, silicon dioxide.
- Phosphoric acid is used as a flux by hobbyists (such as model railroaders) as an aid to soldering.
- Phosphoric acid is also used in hydroponics to lower the pH of nutrient solutions. While other types of acids can be used, phosphorus is a nutrient used by plants, especially during flowering, making phosphoric acid particularly desirable. General Hydroponics pH Down liquid solution contains phosphoric acid in addition to citric acid and ammonium bisulfate with buffers to maintain a stable pH in the nutrient reservoir.
- Phosphoric acid is used as a pH adjuster in cosmetics and skin care products.[3]
- Phosphoric acid is used as an chemical oxidizing agent for activated carbon production.[4]
Biological effects on bone calcium
Phosphoric acid, used in many soft drinks (primarily cola), has been linked to lower bone density in epidemiological studies. For example a study[5] using dual-energy X-ray absorptiometry rather than a questionnaire about breakage, provides reasonable evidence to support the theory that drinking cola results in lower bone density. This study was published in the American Journal of Clinical Nutrition. A total of 1672 women and 1148 men were studied between 1996 and 2001. Dietary information was collected using a food frequency questionnaire that had specific questions about the number of servings of cola and other carbonated beverages and that also made a differentiation between regular, caffeine-free, and diet drinks. The paper finds statistically significant evidence to show that women who consume cola daily have lower bone density. The study also suggests that further research is needed to confirm the findings.
On the other hand, a study funded by Pepsi suggests that low intake of phosphorus leads to lower bone density. The study does not examine the effect of phosphoric acid, which binds with magnesium and calcium in the digestive tract to form salts that are not absorbed, but rather, it studies general phosphorus intake.[6]
However, a controlled, clinical study by Heaney and Rafferty using calcium-balance methods found no impact of carbonated soft drinks containing phosphoric acid on calcium excretion. [7] The study compared the impact of water, milk and various soft drinks (two with caffeine and two without; two with phosphoric acid and two with citric acid)on the calcium balance of 20- to 40-year-old women who customarily consumed ~3 or more cups (680 ml) of a carbonated soft drink per day. They found that, relative to water, only milk and the two caffeine-containing soft drinks increased urinary calcium, and that the calcium loss associated with the caffeinated soft drink consumption was about equal to that previously found for caffeine alone. Phosphoric acid without caffeine had no impact on urine calcium, nor did it augment the urinary calcium loss related to caffeine. Because studies have shown that the effect of caffeine is compensated for by reduced calcium losses later in the day [8], Heaney and Rafferty concluded that the net effect of carbonated beverages – including those with caffeine and phosphoric acid—is negligible and that the skeletal effects of carbonated soft drink consumption are likely due primarily to milk displacement.
Other chemicals such as caffeine (also a significant component of popular common cola drinks) were also suspected as possible contributors to low bone density, due to the known effect of caffeine on calciuria. One other study, comprised of 30 women over the course of a week suggests that phosphoric acid in colas has no such effect, and postulates that caffeine has only a temporary effect which is later reversed. The authors of this study conclude that the skeletal effects of carbonated beverage consumption are likely due primarily to milk displacement.[9] (Another possible confounding factor may be an association between high soft drink consumption and sedentary lifestyle.)
Orthophosphoric acid is the simplest compound of a series known as "phosphoric acids." Two or more orthophosphoric acid molecules can be joined by what is called a "condensation reaction," involving the elimination of water molecules. The products are called polyphosphoric acids.
orthophosphoric acid
H3PO4
When two orthophosphoric acid molecules are condensed into one molecule, pyrophosphoric acid (H4P2O7) is obtained as follows:
- 2 H3PO4 → H4P2O7 + H2O
Likewise, three orthophosphoric acid molecules can condense in a row to obtain tripolyphosphoric acid (H5P3O10). This condensation process can continue with additional orthophosphoric acid units to obtain tetrapolyphosphoric acid (H6P4O13, pictured), and so on. Polyphosphoric acid molecules can have dozens of such phosphoric units bonded in a row. The chemical structures of the first few of these compounds are shown in the above illustration.
Note that each extra phosphoric unit adds 1 extra H (hydrogen) atom, 1 extra P (phosphorus) atom, and 3 extra O (oxygen) atoms. The "backbone" chain of these types of molecules consists of alternating P and O atoms covalently bonded together. A general formula for such poly-acid compounds is HO(PO2OH)xH, where x = number of phosphoric units in the molecule. The four oxygen atoms bonded to each phosphorus atom are in a tetrahedral configuration with the phosphorus in the center of the tetrahedron and the oxygens in each of the four corners.
Notes
- ↑ phosphoric acid. The Columbia Encyclopedia, Sixth Edition. 2001-05. Bartleby.com.Retrieved January 18, 2008.
- ↑ Abdullah Rathur
- ↑ Cosmeticcop [1].Retrieved January 18, 2008.
- ↑ C. Toles, S. Rimmera and J. C. Hower. Production of activated carbons from a washington lignite using phosphoric acid activation. Carbon 34(11) (1996): 1419-1426 [2].Retrieved January 18, 2008.
- ↑ Tucker et al. Am. J Clin. Nut. Oct 2006. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study..Retrieved January 18, 2008.
- ↑ S. Elmståhl, B. Gullberg, L. Janzon , et al. Increased incidence of fractures in middle-aged and elderly men with low intakes of phosphorus and zinc. Osteoporos Int (1998) 8: 333–340.
- ↑ Robert P Heaney and Karen Rafferty, Carbonated beverages and urinary calcium excretion, American Journal of Clinical Nutrition 74 (3)(September 2001): 343-347
- ↑ M.J. Barger-Lux, R.P. Heaney, M.R. Stegman.[3], Effects of moderate caffeine intake on the calcium economy of premenopausal women. American Journal of Clinical Nutrition 52: 722–725
- ↑ Robert P. Heaney and Karen Rafferty, Carbonated beverages and urinary calcium excretion, American Journal of Clinical Nutrition 74 (3): 343-347, (September 2001)
ReferencesISBN links support NWE through referral fees
- Chang, Raymond. 2006. Chemistry, 9th ed. New York: McGraw-Hill Science/Engineering/Math. ISBN 0073221031
- Cotton, F. Albert, and Geoffrey Wilkinson. 1980. Advanced Inorganic Chemistry, 4th ed. New York: Wiley. ISBN 0471027758.
- McMurry, J., and R.C. Fay. 2004. Chemistry, 4th ed. Upper Saddle River, NJ: Prentice Hall. ISBN 0131402080.
External links
All links retrieved November 23, 2022.
- NIOSH Pocket Guide to Chemical Hazards
- Excel spreadsheet containing phosphoric acid titration curve, distribution diagram and buffer pH calculation
| Functional group | |
|---|---|
| Chemical class: Alcohol • Aldehyde • Alkane • Alkene • Alkyne • Amide • Amine • Azo compound • Benzene derivative • Carboxylic acid • Cyanate • Disulfide • Ester • Ether • Haloalkane • Imine • Isocyanide • Isocyanate • Ketone • Nitrile • Nitro compound • Nitroso compound • Peroxide • Phosphoric acid • Pyridine derivative • Sulfone • Sulfonic acid • Sulfoxide • Thioester • Thioether • Thiol |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.