Coral
| Corals | ||||||
|---|---|---|---|---|---|---|
Brain Coral, Diploria labyrinthiformis | ||||||
| Scientific classification | ||||||
| ||||||
| Subclasses | ||||||
|
Alcyonaria |
Corals are those marine invertebrates of the phylum Cnidaria and the class Anthozoa that have external or internal calcareous skeletons. The skeletons of these animals are also called coral. Corals exist as small polyps (as with other Cnidaria), typically in colonies of many individuals and commonly attached to a hard surface. They are closely related to the sea anemones, which are also classified in the Anthozoa class, but which belong to the Actiniaria order.
Although corals can catch plankton and sea anemones can catch fish and other prey items, these animals obtain much of their nutrients from symbiotic unicellular dinoflagellates (type of photosynthetic algae) called zooxanthellae. Consequently, most corals are dependent upon sunlight and for that reason are usually found not far beneath the surface, although in clear waters corals can grow at depths of up to 60 m (200 ft). Other corals, notably the genus Lophelia, do not have associated algae, and can live in much deeper water, with recent finds as deep as 3000 meters (Squires 1959). Corals breed by spawning, with many corals of the same species in a region releasing gametes simultaneously over a period of one to several nights around a full moon.
Corals are major contributors to the physical structure of coral reefs that develop only in tropical and subtropical waters. The most extensive development of extant coral reef is the Great Barrier Reef off the coast of Queensland, Australia. Indonesia is home to almost 600 of the world's approximately 800 known coral reef-building coral species.
Some corals exist in cold waters, such as off the coast of Norway (north to at least 69° 14.24' N) and the Darwin Mounds off western Scotland. Lophelia is a genus of cold-water species.
Corals provide important external and internal values. Externally, they fulfill key roles in food chains and the coral reefs are important habitats for other sea life. Furthermore, an estimated one million people live on coral islands built up from the skeletal remains of corals. However, corals also touch upon the inner aspect of humans. The colors and forms of both the coral organisms and coral structures are a source of beauty to people, and the rich diversity of organisms in the coral reefs and the symbiosis between corals and algae reflects on the harmony of creation. Despite these values, coral reefs are being degraded through human action, either through direct physical damage or as a result of environmentally deleterious actions such as dumping of sewage, or other acts of pollution.
Classification
Corals and sea anemones are part of the Anthozoa, which is a class within the invertebrate phylum Cnidaria. The name of the phylum comes from cnidocytes, which are specialized cells that carry stinging organelles. Other cnidarians are jellyfish, sea pens, sea pansies, sea wasps, and tiny freshwater hydra, among others. Sea pens and sea pansies are also considered to be coral.
Anthozoa can be divided into two groups (Fautin and Romano 2000): Alcyonaria and Zoantharia. Both subclasses contain species known as corals. The Zoantharia also includes the sea anemones (Order Actiniaria) and the tube-dwelling anemones (Order Ceriantharia), among others. Polyps in the subclass Zoantharia without skeletons are generally termed anemones.
The corals are classified into orders as follows (Chen et al. 1995, France et al. 1996, Myers et al. 2006):
- Subclass Alcyonaria (= Octocorallia) (eight tentacles)
- Alcyonacea (soft corals)
- Gorgonacea (sea fans, sea feathers)
- Helioporacea (Indo Pacific blue coral)
- Pennatulacea (sea pens and sea pansies)
- Stolonifera (organ pipe coral)
- Subclass Zoantharia (= Hexacorallia) (more than 8 tentacles - typically 12)
- Antipatharia (black corals, thorny corals)
- Scleractinia (=Madreporaria) (stony corals)
- Corallimorpharia
- Ptychodactiaria
- Extinct orders, from the Paleozoic (570-245 mya) (Oliver 1996):
- Rugosa
- Kilbuchophyllida
- Cothoniida
- Tabulata
- Tabulacondia
- Heliolitida
- Heterocorallida
- Numidiaphyllida
Corals include the important reef builders known as hermatypic corals, found in tropical oceans, and belonging to the subclass Zoantharia of order Scleractinia. The latter are also known as stony corals since the living tissue thinly covers a skeleton composed of calcium carbonate. A coral "head" is formed of thousands of individual polyps, each polyp only a few millimeters in diameter. The colony of polyps function as a single organism by sharing nutrients via a well-developed gastrovascular network. Genetically, the polyps are clones, each having exactly the same genome. Each polyp generation grows on the skeletal remains of previous generations, forming a structure that has a shape characteristic of the species, but also subject to environmental influences.
Anatomy
Theoretically, members of Cnidaria have life cycles that alternate between asexual polyps (the body as a vase shaped form), and sexual, free-swimming forms called medusae (singular medusa; the body in a bell-shaped form). The Anthozoa live only as polyps. Unlike medusae, polyps generally are anchored to the substrate by their basal discs, although a few species can move in curious slow-motion somersaults. By nature, they display their tentacles upwards, away from the substrate. Polyps often live in large colonies.
What we see as a coral is an assemblage of many individual, yet genetically identical, polyps. The polyps are multicellular organisms that feed on a variety of small organisms, from microscopic zooplankton to small fish.
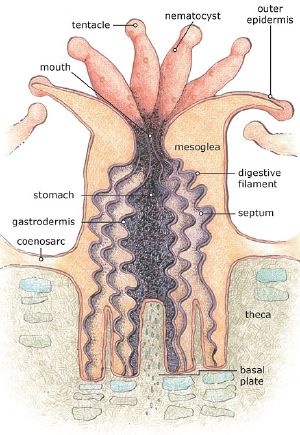
Polyps are usually a few millimetres in diameter, and are formed by a layer of outer epithelium and inner jellylike tissue known as the mesoglea. They are radially symmetrical with tentacles surrounding a central mouth, the only opening to the stomach or coelenteron, through which both food is ingested and waste expelled.
The stomach closes at the base of the polyp, where the epithelium produces an exoskeleton called the basal plate or calicle (L. small cup). This is formed by a thickened calciferous ring (annular thickening) with six supporting radial ridges (as shown below). These structures grow vertically and project into the base of the polyp allowing it to retreat into the exoskeleton for protection.
The polyp grows by vertical extension of the basal plate forming vertical calices, which are occasionally septated to form a new, higher, basal plate. Over many generations this extension forms the large calciferous structures of corals and ultimately coral reefs.
Formation of the calciferous exoskeleton involves deposition of calcium carbonate by the polyps from calcium ions that accumulate from seawater. The rate of deposition, while varying greatly between species and environmental conditions, can be as much as 10 g / m² of polyp / day (0.3 ounce / sq yd / day). This is however dependent on [[light, with production reduced by 90 percent at night compared to the middle of the day (Marine Reef 2006).
The polyp's tentacles trap prey using stinging cells called nematocysts. These are cells modified to capture and immobilize prey such as plankton, by injecting poisons, firing very rapidly in response to contact. In fire corals, these poisons are harmful to humans; however, in most other cases it is harmless. Nematocysts can also be found in jellyfish and sea anemones. After the toxins injected by nematocysts immobilise or kill prey, the prey can then be drawn into the polyp's stomach by the tentacles through a contractile band of epithelium called the pharynx.
Aside from feeding on plankton, corals belong in a symbiotic relationship with a class of algae, zooxanthellae. Typically a polyp will harbor particular species of algae, which will photosynthesise and thereby provide energy for the coral and aid in calcification (Madl and Yip 2000). Meanwhile, the algae live in a safe environment and use the carbon dioxide and nitrogenous waste produced by the polyp. Due to the strain the algae can put on the polyp, stress on the coral often triggers ejection of the algae, known on a large scale as coral bleaching as it is the algae that gives coral color. This allows the polyp to live longer during stressful periods, and to regain the algae at a later time; however if the conditions persist the polyps and corals die without the photosynthetic algae (Toller et al. 2001).
The polyps are interconnected by a complex and well developed system of gastrovascular canals allowing significant sharing of nutrients and symbiotes. In soft corals, these have been found to range in size from 50-500 μm in diameter and to allow transport of both metabolites and cellular components (Gateno 1998).
Reproduction
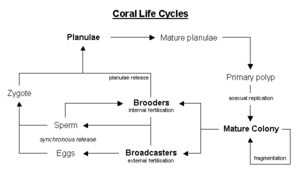
Corals reproduce predominantly sexually, with 25 percent of hermatypic corals (stony corals) forming single sex (gonochoristic) colonies and the rest hermaphroditic (Veron 2000).
About 75 percent of all hermatypic corals release gametesâeggs and spermâinto the water to spread colonies over large distances in what is called broadcast spawning. The gametes fuse during fertilization to form a microscopic larva called a planula, typically pink and elliptical in shape. A moderately sized coral colony can form several thousands of these larva per year to overcome the huge hazards that prevent formation of a new colony (Barnes and Hughes 1999).
Corals that do not broadcast spawn are called brooders, with most non-stony corals displaying this characteristic. These corals release sperm, but keep the eggs, allowing larger, negatively buoyant, planulae to form, which are later released ready to settle (Madl and Yip 2000).
The planula swims towards light, positive phototaxis, to surface waters where it drifts for a time and then swims back down to locate a surface on which it can attach and establish a new colony. The time from spawning to settling is often two to three days, but can be up to two months (Jones and Endean 1973).
The larva grows into a coral polyp and eventually becomes a coral head by asexual budding and growth to create new polyps.
Synchronous spawning is very typical on a coral reef. Even when there are multiple species present, all the corals on the reef may release gametes during the same night. This synchrony is essential so that male and female gametes can meet and form planula. The cues that guide the release are complex, but over the short term appear to involve lunar changes and time of sunset, although chemical signalling has not been ruled out (Veron 2000). Synchronous spawning may have the result of forming coral hybrids, perhaps involved in coral speciation (Hatta et al. 1999).
In some places, the coral spawn can be dramatic, usually occurring at night, where the usually clear water becomes cloudy with gametes.
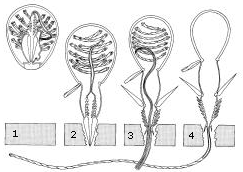
Within a head of coral, the genetically identical polyps reproduce asexually to allow growth of the colony. This is achieved either through gemmation or budding, or through division, both shown in the diagrams of Orbicella annularis on the right. Budding involves a new polyp growing from an adult, whereas division forms two polyps as large as the original (Barnes 1999).
Whole colonies can reproduce asexually through fragmentation, where a piece broken off a coral head and moved by wave action can continue to grow in a new location.
Geological history
Although corals first appeared in the Cambrian period, some 570 million years ago, they are extremely rare as fossils until the Ordovician period, when Rugose and Tabulate corals became widespread.
Tabulate corals occur in the limestones and calcareous shales of the Ordovician and Silurian periods, and often form low cushions or branching masses alongside Rugose corals. Their numbers began to decline during the middle of the Silurian period and they finally became extinct at the end of the Permian period. The skeletons of Tabulate corals are composed of a form of calcium carbonate known as calcite.
Rugose corals became dominant by the middle of the Silurian period, and became extinct early in the Triassic period. The Rugose corals may be either solitary or colonial, and like the Tabulate corals their skeletons are also composed of calcite. The finest details of their skeletal structures are often well preserved, and such fossils may be cut and polished.
Scleractinian corals diversified during the Mesozoic and Cenozoic eras and are at the height of their development today. Their fossils may be found in small numbers in rocks from the Triassic, and they are relatively common fossils in rocks from the Jurassic and Cretaceous periods as well as the Cenozoic era. The skeletons of Scleractinian corals are composed of a form of calcium carbonate known as aragonite. Although they are geologically younger than the Tabulate and Rugose corals, the aragonite skeleton of scleractinian corals does not tend to preserve well, so it is often easier to find fossils of the more ancient Tabulate and Rugose corals.
At certain times in the geological past, corals were very abundant, just as modern corals are in the warm clear tropical waters of certain parts of the world today. And like modern corals, their fossil ancestors built reefs beneath the ancient seas. Some of these reefs now lie as great structures in the midst of sedimentary rocks. Such reefs can be found in the rocks of many parts of the world including those of the Ordovician period of Vermont in the United States, the Silurian period of the Michigan Basin and in many parts of Europe, the Devonian period of Canada and the Ardennes in Belgium, and the Cretaceous period of South America and Denmark. Reefs from both the Silurian and Carboniferous periods have been recorded as far north as Siberia, and as far south as Australia.

However, these ancient reefs are not composed entirely of corals. Algae and sponges, as well as the fossilized remains of many echinoids, brachiopods, bivalves, gastropods, and trilobites that lived on the reefs help to build them. These fossil reefs are prime locations where fossils of many different types are found, in addition to the corals themselves.
Corals are not restricted to just reefs, many solitary corals may be found in rocks where reefs are not present (such as Cyclocyathus which occurs in the Cretaceous period Gault clay formation of England).
As well as being important rock builders, some corals are useful as zone (or index) fossils, enabling geologists to date the age the rocks in which they are found, particularly those found in the limestones of the Carboniferous period.
Environmental effects on coral
Coral can be sensitive to environmental changes, such as changes in nutrients, temperature, and pollution, as well as physical damage related to tourism and fishing.
A coral reef can easily be swamped in algae if there are too many nutrients in the water. Some reefs, such as those off the coast of Tobago, have been threatened by the runoff of sewage adding extra nurtrients into the ocean. Measures to rectify this include sewage treatment and discharge of effluent into the open ocean beyond the reefs. Coral will also die if the water temperature changes by more than a degree or two beyond its normal range or if the salinity of the water drops. Climatic variations, such as El Niño-Southern Oscillation (ENSO), can cause the temperature changes that destroy corals. For example, the hydrocoral Millepora boschmai, located on the north shore of Uva Island (named Lazarus Cove), Gulf of ChiriquÃ, Panamá, survived the 1982-1983 ENSO warming event, but during the 1997-1998 ENSO all the surviving colonies bleached and died six years later (Glynn 2001). In an early symptom of environmental stress, corals expel their zooxanthellae; without their symbiotic unicellular algae, coral tissues then become colorless as they reveal the white of their calcium carbonate skeletons, an event known as coral bleaching (Hoegh-Guldberg 1999).
Another problem is removal of coral from reefs by divers taking pieces of coral. Many governments now prohibit this. However, this does not stop damage done by "reef walking" (snorkelers walking on the coral) or anchors dropped by dive boats or fishermen.
A combination of temperature changes, pollution, and overuse by divers and jewelry producers has led to the destruction of many coral reefs around the world. Because of the various stresses, some scientists are predicting that over 50 percent of the coral reefs in the world may be destroyed or vanish by the year 2030 (Norlander 2003).
Legal and educational efforts are being made to counteract the reef damage. In places where local fishing causes reef damage, such as the island of Rodrigues, education schemes have been run to educate the population about reef protection and ecology. Tour operators, who take scuba divers and snorkelers to visit the reefs, are being educated regarding the care of the reefs as well.
Coral in history and mythology
The origin of coral is explained in Greek mythology by the story of Perseus. Having petrified the sea monster threatening Andromeda (Cetus or Tiamat, depending on the source), Perseus placed Medusa's head on the riverbank while he washed his hands. When he recovered her head, he saw that her blood had turned the seaweed (sometimes the reeds) into coral. Thus, the Greek word for coral is "Gorgeia," as Medusa was one of the three fearsome female Gorgons with snakes for hair. Poseidon resided in a palace made of coral and gems, and Hephaestus first crafted his work from coral.
The Ancient Romans believed coral could protect children from harm, as well as cure wounds made by snakes and scorpions and diagnose diseases by changing color. Pliny has recorded the trade of coral between the Mediterranean and India in the first century C.E.
There is a widespread current myth that coral debris in a wound will continue to grow. That is not true; the temperature and other conditions in a human body will very quickly kill the delicate coral polyps. The myth may stem from tiny chunks of coral in a wound taking a long time to be expelled, giving the impression that they grew there. However, infection by bacteria from sea water is a serious danger of coral wounds, and for this reason, they should be thoroughly cleaned.
Uses
Ancient coral reefs now on land are often mined for limestone or building blocks ("coral rag"). An example of the former is the quarrying of Portland limestone from the Isle of Portland. Coral rag is an important local building material in places such as the east African coast.
Reddish coral is sometimes used as a gemstone, especially in Tibet. Pure red coral is known as 'fire coral' and it is very rare because of the demand for perfect fire coral for jewelery-making purposes.
Local economies near major coral reefs benefit from recreational scuba diving and snorkeling tourism; however, this also has deleterious implications such as removal or accidental destruction of coral. Coral reefs also provide a rich fishing environment.
Some coral species exhibit banding in their skeletons resulting from annual variations in their growth rate. In fossil and modern corals, these bands allow geologists to construct year-by-year chronologies, a kind of incremental dating, which combined with geochemical analysis of each band, can provide high-resolution records of paleoclimatic and paleoenvironmental change (Schrag and Linsley 2002).
Certain species of corals form communities called microatolls. The vertical growth of microatolls is limited by average tidal height. By analyzing the various growth morphologies, microatolls can be used as a low resolution record of patterns of sea level change. Fossilized microatolls can also be dated using radioactive carbon dating to obtain a chronology of patterns of sea level change. Such methods have been used to used to reconstruct Holocene sea levels (Smithers and Woodroffe 2000).
ReferencesISBN links support NWE through referral fees
- Barnes, R., and R. Hughes. 1999. An Introduction to Marine Ecology, 3rd ed.. Malden, MA: Blackwell Science, Inc. ISBN 0865428344
- Chen, C. A., D. M. Odorico, M. Ten Lohuis, J. E. N. Veron, and D. J. Miller. 1995. Systematic relationships within the Anthozoa (Cnidaria: Anthozoa) using the 5'-end of the 28S rDNA. Molecular Phylogeny and Evolution 4(2): 175-183. PMID:7663762.
- Fautin, D. G., and S. L. Romano. 2000. Anthozoa: Sea Anemones, Corals, Sea Pens. The Tree of Life Web Project.
- France, S. C., P. E. Rosel, J. E. Agenbroad, L. S. Mullineaux, and T. D. Kocher. 1996. "DNA sequence variation of mitochondrial large-subunit rRNA provides support for a two subclass organization of the Anthozoa (Cnidaria)." Molecular Marine Biology and Biotechnology 5(1):15-28. PMID:8869515.
- Gateno, D., A. Israel, Y. Barki, and B. Rinkevich. 1998. Gastrovascular circulation in an octocoral: Evidence of significant transport of coral and symbiont cells. The Biological Bulletin 194(2): 178-186.
- Glynn, P. 2001. "History of significant coral bleaching events and insights regarding amelioration." In R. V. Salm and S. L. Coles, editors. 2001. Coral Bleaching and Marine Protected Areas: Proceedings of the Workshop on Mitigating Coral Bleaching Impact Through MPA Design, 36-39. Bishop Museum, Honolulu, Hawaii, May 29-31, 2001. Asia Pacific Coastal Marine Program Report #0102, The Nature Conservancy, Honolulu, Hawaii, USA. Online PDF fulltext version
- Hatta, M., H. Fukami, W. Wang, M. Omori, K. Shimoike, T. Hayashibara, Y. Ina, and T. Sugiyama. 1999. "Reproductive and genetic evidence for a reticulate evolutionary theory of mass spawning corals." Molecular Biology and Evolution 16(11): 1607-1613. PMID:8096089.
- Hoegh-Guldberg, O. 1999. "Climate change, coral bleaching and the future of the world's coral reefs." Marine and Freshwater Research 50(8):839-866.
- Jones, O.A., and R. Endean. 1973. Biology and Geology of Coral Reefs. New York, NY: Harcourt Brace Jovanovich. ISBN 0123896029
- Madl, P. and M. Yip. 2000. Field Excursion to Milne Bay Province: Papua New Guinea]. (accessed March 31, 2006).
- Marine Reef. 2006. Anatomy of Coral. (accessed March 31, 2006).
- Myers, P., R. Espinosa, C. S. Parr, T. Jones, G. S. Hammond, and T. A. Dewey. 2006. Subclass Alcyonaria. The Animal Diversity Web (online). (accessed Marcy 31, 2006).
- Norlander. 2003. Coral crisis! Humans are killing off these bustling underwater cities. Can coral reefs be saved?. Science World: December 8, 2003.
- Oliver, W. A., Jr. 1996. "Origins and relationships of Paleozoic coral groups and the origin of the Scleractinia." In G. D. J. Stanley (ed.), Paleobiology and Biology of Corals.: 107-134. Columbus, Ohio: The Paleontological Society.
- Schrag, D. P., and B. K. Linsley. 2002. Corals, chemistry, and climate. Science 296(8):277-278. PMID:11951026.
- Smithers, S. G., and C. D. Woodroffe. 2000. "Microatolls as sea-level indicators on a mid-ocean atoll." Marine Geology 168:61-78.
- Squires, D. F. 1959. "Deep sea corals collected by the Lamont Geological Observatory. 1. Atlantic corals." Am. Mus. Nov. 1965: 1â42.
- Toller, W. W., R. Rowan, and N. Knowlton. 2001. Repopulation of Zooxanthellae in the Caribbean corals Montastraea annularis and M. faveolata following experimental and disease-associated bleaching. The Biological Bulletin 201: 360-373.
- Veron, J. 2000. Corals of the World. Volume 3, 3rd Edition. Australia: Australian Institute of Marine Sciences and CRR Qld Pty Ltd. ISBN 0865428344
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.