Ammonium sulfate
| Ammonium sulfate | |
|---|---|
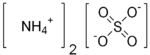
| |
| IUPAC name | Ammonium sulfate |
| Other names | ammonium sulfate (2:1); diammonium sulfate; sulfuric acid diammonium salt; mascagnite; Actamaster; Dolamin |
| Identifiers | |
| CAS number | [] |
| SMILES | [O-]S([O-])(=O)=O.[NH4+].[NH4+] |
| Properties | |
| Molecular formula | (NH4)2SO4 |
| Molar mass | 132.14 g/mol |
| Appearance | Fine white hygroscopic granules or crystals. |
| Density | 1.77 g/cm³ @ 50 °C (122 °F) |
| Melting point |
235-280 °C, 508-553 K, 455-536 °F (decomposes) |
| Solubility in water | 70.6 g/100 mL (0 °C) and 103.8 g/100 mL (100 °C)[1] |
| Critical relative humidity | 79.2% at 30 °C |
| Related Compounds | |
| Related compounds | Ammonium iron sulfate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Ammonium sulfate is an inorganic chemical compound with the chemical formula (NH4)2SO4. It contains 21 percent nitrogen in the form of ammonium ions and 24 percent sulfur as sulfate ions. The purified material takes the form of white granules or crystals. It is commonly used as a fertilizer and as an agricultural spray adjuvant for water soluble pesticides. It is also used in the preparation of other ammonium salts.
Occurrence in nature
Ammonium sulfate occurs naturally as the rare mineral mascagnite in volcanic fumaroles and due to coal fires on some dumps.[2]
Properties
Ammonium sulfate is a salt of ammonia and sulfuric acid, and its chemical formula is (NH4)2SO4. Under standard conditions of temperature and pressure, it takes the form of fine white granules or crystals. It is not soluble in alcohol or liquid ammonia. It is slightly hygroscopic, absorbing water from the air at relative humidity above 81 percent (at about 20 °C).
Synthesis
Ammonium sulfate is prepared commercially by reacting ammonia with sulfuric acid (H2SO4). Ammonium sulfate is prepared commercially from the ammoniacal liquor of gas-works and is purified by recrystallization. It forms large, rhombic prisms, has a somewhat saline taste and is easily soluble in water. The aqueous solution on boiling loses some ammonia and forms an acid sulfate.
Uses
Ammonium sulfate is used largely as an artificial fertilizer for alkaline soils. In the soil, the sulfate ion is released and forms sulfuric acid, lowering the pH balance of the soil (as do other sulfate compounds such as aluminum sulfate), while contributing essential nitrogen for plant growth.
In addition, it is used as an agricultural spray adjuvant for water soluble insecticides, herbicides, and fungicides. There it functions to bind iron and calcium cations that are present in both well water and plant cells. It is particularly effective as an adjuvant for 2,4-D (amine), glyphosate, and glufosinate herbicides.
It is also used in the preparation of other ammonium salts.
In biochemistry, ammonium sulfate precipitation is a common method for purifying proteins by precipitation. As such, ammonium sulfate is also listed as an ingredient in many vaccines used in the United States, according to the Centers for Disease Control and Prevention (CDC).[3]
Ammonium sulfate is also a food additive.[4]
See also
Notes
- ↑ Handbook of Chemistry and Physics
- ↑ Mascagnite Mindat.org. Retrieved October 9, 2008.
- ↑ Vaccine Excipient & Media Summary, Part 2 Centers for Disease Control and Prevention. Retrieved October 9, 2008.
- ↑ Lower-Carb Italian Herb Bread panerabread.com.
ReferencesISBN links support NWE through referral fees
- Brown Jr., Theodore L., H. Eugene LeMay, Bruce Edward Bursten, and Julia R. Burdge. 2002. Chemistry: The Central Science, 9th ed. Upper Saddle River, NJ: Prentice Hall. ISBN 0130669970
- Chang, Raymond. 2006. Chemistry, 9th ed. New York, NY: McGraw-Hill Science/Engineering/Math. ISBN 0073221031
- Havlin, John L., Samuel L. Tisdale, James D. Beaton, and Werner L. Nelson. 2004. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 7th ed. Upper Saddle River, NJ: Pearson Prentice Hall. ISBN 0130278246
- International Fertilizer Development Center, and United Nations Industrial Development Organization. 1998. Fertilizer Manual. Dordrecht: Kluwer Academic. ISBN 0792350324
- Moore, John W., Conrad L. Stanitski, and Peter C. Jurs. 2005. Chemistry: The Molecular Science, 2nd ed. Belmont, CA: Thompson Brooks/Cole. ISBN 9780534422011
- Walters, Charles. 2003. Eco-farm: An Acres U.S.A. Primer. Austin, TX: Acres U.S.A. ISBN 0911311742
External links
All links retrieved July 25, 2023.
- Ammonium Sulfate (Precipitation) Calculator EnCor Biotechnology Inc.
- Using Ammonium Sulfate Fertilizer as an Organic Mulch Fire Retardant (A Research Note) Urban Forestry South.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.