Osmosis
Osmosis is the movement of water molecules across a semi-permeable membrane from a region of low solute potential to an area of high solute potential (or equivalently, from a region of high solvent potential to a region of low solvent potential). The partially permeable membrane must be permeable to the solvent (the solution), but not to the solute (the substance dissolved in the solution), resulting in a pressure gradient across the membrane.
Osmosis is an important topic in biology because it provides the primary means by which water is transported into and out of cells. Human creativity has also learned to take advantage of principles related to osmosis for such useful processes as desalination, water purification, water treatment, and food processing.
Water molecules travel through the cell membrane/tonoplast/protoplast in two ways, either by diffusing across the phospholipid bilayer directly, or via aquaporins (small transmembrane proteins similar to those in facilitated diffusion and in creating ion channels).
Osmosis is a natural phenomenon. However, it can be artificially opposed by increasing the pressure in the section of high solute concentration with respect to that in the low solute concentration. The osmotic pressure is equal to the force per unit area that is necessary to prevent passage of solvent into the region of greater solvent concentration. The osmotic pressure depends on the concentration of the solvent, not its identity.
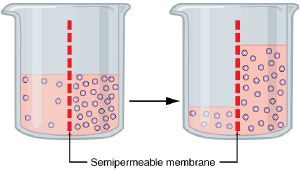
Basic explanation of osmosis
Consider a permeable membrane with apertures small enough to allow water molecules, but not larger molecules, to pass through.
Suppose this semi-permeable (selectively permeable) membrane is in a volume of pure water, that is, separating two regions of pure water. At a molecular scale, every time a water molecule hits the membrane (due to their natural kinetic energy), it has a defined likelihood of passing through. In this case, since the circumstances on both sides of the membrane are equivalent, there is no net flow of water through it.
However, if there is a solution on one side of the semi-permeable membrane, that side will have fewer water molecules and thus fewer collisions with the membrane. This will result in a net flow of water to the side with the solution.
Assuming the membrane does not break, this net flow will slow and finally stop as the pressure on the solution side becomes such that the movement in each direction is equal. (Whether that be due to a natural equilibrium or not, osmosis is inhibited by factors such as pressure potential or osmotic pressure.)
Osmosis can also be explained via the notion of entropy, from statistical mechanics. As above, suppose a semi-permeable membrane separates equal amounts of pure solvent and a solution. Since a solution possesses more entropy than pure solvent, the second law of thermodynamics states that solvent molecules will flow into the solution until the entropy of the combined system is maximized. Notice that, as this happens, the solvent loses entropy while the solution gains entropy. Equilibrium, hence maximum entropy, is achieved when the entropy gradient becomes zero.
Examples of osmosis
Many plant cells perform osmosis. The osmotic entry of water from outside the cell is opposed and eventually equaled by the pressure exerted by the cell wall, creating a steady state. In fact, osmotic pressure is the main cause of support in plant leaves. In other words, the plant takes in the water so it can stay alive.
The terms hypotonic, isotonic, or hypertonic reference the concentration of an external solution relative to the cell. If a plant cell or an animal cell is placed in a solution of sugar or salt:
- If the medium surrounding the cell has a higher water concentration than the cell (less concentrated in terms of solutes), the cell will gain water through osmosis. Such a solution is called a hypotonic solution.
- If the medium has exactly the same water concentration, there will be no net movement of water across the cell membrane. Such a solution is called a isotonic solution.
- If the medium has a lower concentration of water than the cell, meaning that it is a more concentrated solution, the cell will lose water by osmosis. Such a solution is called a hypertonic solution.
When a plant cell is placed in a hypertonic solution, the water in the cell moves to an area higher in solute concentration, and the cell shrinks and so becomes flaccid (pronounced flaxid). This means the cell has become plasmolysed— that is, the cell membrane (plasma membrane) has completely separated from the cell wall due to lack of water pressure on it (the opposite of turgid).
Osmosis can also be seen very effectively when potato slices are added to a high concentration of salt solution. The water from inside the potato moves to the salt solution, causing the potato to shrink and to lose its “turgor pressure” (osmotic pressure). The more concentrated the salt solution, the bigger the difference in size and weight of the potato slice.
In unusual environments, osmosis can be very harmful to organisms. For example, freshwater and saltwater aquarium fish placed in water with a different salt level (than they are adapted to) will die quickly, and in the case of saltwater fish rather dramatically. By the same principle, table salt is useful to kill leeches and slugs.
Osmotic pressure
As noted above, osmosis can be opposed by increasing the pressure in the region of high solute concentration with respect to that in the low solute concentration region. The force per unit area, or pressure, required to prevent the passage of water through a selectively permeable membrane and into a solution of greater concentration is equivalent to the osmotic pressure of the solution, or turgor. Osmotic pressure is a colligative property, meaning that the property depends on the concentration of the solute but not on the type of solute.
Increasing the pressure increases the chemical potential of the system in proportion to the molar volume (). Therefore, osmosis stops when the increase in potential due to pressure equals the potential decrease from equation 1, i.e.:
Where is the osmotic pressure and is the molar volume of the solvent.
For the case of very low solute concentrations, -ln(1-) ≈ and equation 2 can be rearranged into the following expression for osmotic pressure:
Reverse and forward osmosis and applications
Reverse osmosis. The osmosis process can be driven in reverse with solvent moving from a region of high solute concentration to a region of low solute concentration by applying a pressure in excess of the osmotic pressure. The reverse osmosis technique is commonly applied in desalination, water purification, water treatment, and food processing. Recent advances in pressure exchange and the ongoing development of low pressure membranes have significantly reduced the costs of water produced by reverse osmosis.
Forward osmosis. Osmosis may be used directly to achieve separation of water from a "feed" solution containing unwanted solutes. A "draw" solution of higher osmotic pressure than the feed solution is used to induce a net flow of water through a semi-permeable membrane, such that the feed solution becomes concentrated as the draw solution becomes dilute. The diluted draw solution may then be used directly (as with an ingestible solute like glucose), or sent to a secondary separation process for the removal of the draw solute. This secondary separation can be more efficient than a reverse osmosis process would be alone, depending on the draw solute used and the feedwater treated. Forward osmosis is an area of ongoing research, focusing on applications in desalination, water purification, water treatment, and food processing.
ReferencesISBN links support NWE through referral fees
- Murad, S., K. Oder, and J. Lin. 1998. "Molecular simulation of osmosis, reverse osmosis and electro-osmosis in aqueous and electrolyte solutions." Molecular Physics 95: 401-408.
- Powles, J. G., and S. Murad. 1998. "The simulation of semi-permeable membranes: Osmosis, reverse osmosis and electro-osmosis in electrolyte solutions." Journal of Molecular Liquids 78: 225-231.
- Powles, J. G., B. Holtz, W. A. B. Evans, and S. Murad. 1997. "Can osmotic pressure be negative?" Molecular Physics 90: 665-670.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.