| |
| |
Aspirin
| |
| Systematic name | |
| IUPAC name 2-(acetyloxy)benzoic acid | |
| Identifiers | |
| CAS number | 50-78-2 |
| ATC code | B01AC06 |
| PubChem | 2244 |
| DrugBank | APRD00264 |
| Chemical data | |
| Formula | C9H8O4 benzene ring |
| Mol. weight | 180.16 g/mol |
| Synonyms | 2-acetyloxybenzoic acid 2-acetoxybenzoic acid acetylsalicylate acetylsalicylic acid O-acetylsalicylic acid |
| Physical data | |
| Density | 1.40 g/cm3 |
| Melt. point | 136°C (277°F) |
| Boiling point | 140°C (284°F) |
| Solubility in water | 4.6 mg/mL (20°C) |
| Pharmacokinetic data | |
| Bioavailability | rapid & complete |
| Protein binding | 99.5 percent |
| Metabolism | hepatic |
| Half life | 300-650mg dose, 3.1 - 3.2 hours 1g dose 5 hours 2g dose 9 hours |
| Excretion | renal |
| Therapeutic considerations | |
| Pregnancy cat. | ? |
| Legal status | ? |
| Routes | oral |
Aspirin or acetylsalicylic acid is a drug in the family of salicylates (carboxylic acid), often used as an analgesic (against minor pains and aches), antipyretic (against fever), and anti-inflammatory (against localized redness, swelling, heat, and pain). It has also an anticoagulant ("blood-thinning") effect and is used in long-term low-doses to prevent heart attacks.
Low-dose, long-term aspirin irreversibly blocks formation of the lipid thromboxane A2 in platelets (type of blood cell involved in blood clotting). This produces an inhibitory effect on platelet aggregation, and this blood-thinning property makes it useful for reducing the incidence of heart attacks. High doses of aspirin are also given immediately after an acute heart attack. These doses may also inhibit the synthesis of prothrombin, a coagulation protein that converts soluble fibrinogen into insoluble strands of fibrin, and thus aspirin may produce a second and different anticoagulant effect.
Several hundred fatal overdoses of aspirin occur annually, but the vast majority of its uses are beneficial. Its primary undesirable side effects, especially in stronger doses, are gastrointestinal distress (including ulcers and stomach bleeding) and tinnitus. Another side effect, due to its anticoagulant properties, is increased bleeding in menstruating women. Because there appears to be a connection between aspirin and Reye's syndrome, aspirin is no longer used to control flu-like symptoms in minors.
While aspirin is a common method of dealing with pain and preventing heart attacks, its side effects dictate that a more comprehensive approach to pain relief and disease prevention should be considered as well. Such a comprehensive approach would include seeking for the underlying causes of a health problem, rather than masking the symptoms; learning to manage stress; and possibly involving lifestyle or diet changes. In addition, there are a multitude of non-drug approaches to preventing and dealing with pain, including such means as acupuncture, herbal medicine, hypnosis, naturopathy, chiropractic, and so forth. Some alternative methods take into account not just the physical aspects of health, but also the mental, emotional, and spiritual aspects of being human.
Aspirin was the first discovered member of the class of drugs known as non-steroidal anti-inflammatory drugs (NSAIDs), not all of which are salicylates, though they all have similar effects and a similar action mechanism.
Aspirin as genericized trademark
The brand name Aspirin was coined by the Bayer Company of Germany. In some countries, the name is used as a generic term for the drug rather than considered as the manufacturer's trademark. In countries in which Aspirin remains a trademark, the abbreviation ASA (for acetylsalicylic acid) is used as a generic term (ASS in German-language countries, for Acetylsalicylsäure; AAS in Spanish- and Portuguese-language countries, for ácido acetilsalicÃlico, and in French-language countries, for acide acétylsalicylique).
The name "aspirin" is composed of a- (from the acetyl group) -spir- (from the spiraea flower) and -in (a common ending for drugs at the time). It has also been known that the name originated by another means: "As" referring to AcetylSalicylic and "pir" in reference to one of the scientists who was able to isolate it in crystalline form, Raffaele Piria, with "in" arising from the same reasons as stated above.
On March 6, 1899, Bayer registered Aspirin as a trademark. However, the German company lost the right to use the trademark in many countries as the Allies seized and resold its foreign assets after World War I. The right to use "Aspirin" in the United States (along with all other Bayer trademarks) was purchased from the U.S. government by Sterling Drug in 1918. However, even before the patent for the drug expired in 1917, Bayer had been unable to stop competitors from copying the formula and using the name elsewhere, and so, with a flooded market, the public was unable to recognize "Aspirin" as coming from only one manufacturer. Sterling was subsequently unable to prevent "Aspirin" from being ruled a genericized trademark in a U.S. federal court in 1921. Sterling was ultimately acquired by Bayer in 1994, but this did not restore the U.S. trademark. Other countries (such as Canada and many countries in Europe) still consider "Aspirin" a protected trademark.
Discovery
Hippocrates, a Greek physician, wrote in the fifth century B.C.E. about a bitter powder extracted from willow bark that could ease aches and pains and reduce fevers. This remedy is also mentioned in texts from ancient Sumeria, Egypt, and Assyria. Native Americans claim to have used it for headaches, fever, sore muscles, rheumatism, and chills. The Reverend Edward Stone, a vicar from Chipping Norton, Oxfordshire, England, noted in 1763 that the bark of the willow was effective in reducing a fever.[1]
The active extract of the bark, called salicin, after the Latin name for the White willow (Salix alba), was isolated to its crystalline form in 1828 by Henri Leroux, a French pharmacist, and Raffaele Piria, an Italian chemist, who then succeeded in separating out the acid in its pure state. Salicin is highly acidic when in a saturated solution with water (pH = 2.4), and is called salicylic acid for that reason.
This chemical was also isolated from meadowsweet flowers (genus Filipendula, formerly classified in Spiraea) by German researchers in 1839. While their extract was somewhat effective, it also caused digestive problems, such as irritated stomach and diarrhea, and even death when consumed in high doses. In 1853, a French chemist named Charles Frederic Gerhardt neutralized salicylic acid by buffering it with sodium (sodium salicylate) and acetyl chloride, creating acetosalicylic anhydride. Gerhardt's product worked, but he had no desire to market it and abandoned his discovery. In 1897, researcher Arthur Eichengrun and Felix Hoffmann, a research assistant at Friedrich Bayer & Co. in Germany, derivatized one of the hydroxyl functional groups in salicylic acid with an acetyl group (forming the acetyl ester), which greatly reduced the negative effects. This was the first synthetic drug, not a copy of something that existed in nature, and the start of the pharmaceutical industry.
Hoffmann made some of the formula and gave it to his father, who was suffering from the pain of arthritis and could not stand the side effects of salicylic acid. With good results, he then convinced Bayer to market the new wonder drug. Aspirin was patented on March 6, 1899. It was marketed alongside another of Hoffmann's products, an acetylated synthetic of morphine called Heroin that he invented 11 days after Aspirin. Heroin was initially the more successful of the two painkillers and it was common belief that it was healthier than Aspirin. But, as Heroin's shortcoming of addictiveness became more obvious, Aspirin stepped to the forefront. Aspirin was originally sold as a powder and was an instant success; in 1915, Bayer introduced Aspirin tablets.
Several claims to invention of acetylsalicylic acid have arisen. Acetylsalicylic acid was already being manufactured by the Chemische Fabrik von Heyden Company in 1897, although without a brand name. Arthur Eichengrün claimed in 1949 that he planned and directed the synthesis of aspirin, while Hoffmann's role was restricted to the initial lab synthesis using Eichengrün's process. In 1999 Walter Sneader of the Department of Pharmaceutical Sciences at the University of Strathclyde in Glasgow reexamined the case and agreed with Eichengrün's account. Bayer continues to recognize Felix Hoffmann as aspirin's official inventor. Despite its argued origin, Bayer's marketing was responsible for bringing it to the world.
It was not until the 1970s that the mechanism of action of aspirin and similar drugs called non-steroidal anti-inflammatory drugs (NSAIDs, such as ibuprofen and aspirin) was elucidated (see below).
Synthesis of aspirin
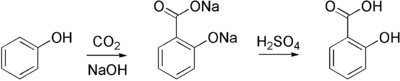
Aspirin is commercially synthesized using a two-step process. First, phenol (originally called carbolic acid and generally extracted from coal tar) is treated with a sodium base generating sodium phenoxide, which is then reacted with carbon dioxide under high temperature and pressure to yield salicylate, which is acidifed, yielding salicylic acid. This process is known as the Kolbe-Schmitt reaction.
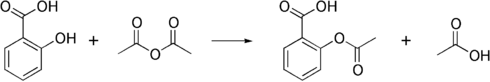
Salicylic acid is then acetylated using acetic anhydride, yielding aspirin and acetic acid as a byproduct. It is a common experiment performed in organic chemistry labs, and generally tends to produce low yields due to the relative difficulty of its extraction from an aqueous state. The trick to getting the reaction to work is to acidify with Phosphoric acid and heat the reagents under reflux with a boiling water bath for between 40 minutes and an hour.
Formulations containing high concentrations of aspirin often smell of vinegar. This is because aspirin can undergo autocatalytic degradation to salicylic acid in moist conditions, yielding salicylic acid and acetic acid.
How it works
In a piece of research for which he was awarded both a Nobel Prize in Physiology or Medicine in 1982 and a knighthood, John Robert Vane, who was then employed by the Royal College of Surgeons in London, showed in 1971 that aspirin suppresses the production of the lipid compounds prostaglandins and thromboxanes. This happens because cyclooxygenase, an enzyme that participates in the production of prostaglandins and thromboxanes, is irreversibly inhibited when aspirin acetylates it. This makes aspirin different from other NSAIDS (such as diclofenac and ibuprofen), which are reversible inhibitors.
Prostaglandins are local hormones (paracrine) produced in the body and have diverse effects in the body, including but not limited to transmission of pain information to the brain, modulation of the hypothalamic thermostat, and inflammation.
Thromboxanes are responsible for the aggregation of platelets that form blood clots. Heart attacks are primarily caused by blood clots, and their reduction with the introduction of small amounts of aspirin has been seen to be an effective medical intervention. The side effect of this is that the ability of the blood in general to clot is reduced, and excessive bleeding may result from the use of aspirin.
More recent work has shown that there are at least two different types of cyclooxygenase: COX-1 and COX-2. Aspirin inhibits both of them. Newer NSAID drugs called COX-2 selective inhibitors have been developed that inhibit only COX-2, with the hope for reduction of gastrointestinal side effects.
However, several of the new COX-2 selective inhibitors have been withdrawn, after evidence emerged that COX-2 inhibitors increase the risk of heart attack. It is proposed that endothelial cells lining the arteries in the body express COX-2, and, by selectively inhibiting COX-2, prostaglandins (specifically PGF2) are downregulated with respect to thromboxane levels, as COX-1 in platelets is unaffected. Thus, the protective anti-coagulative effect of PGF2 is decreased, increasing the risk of thrombus and associated heart attacks and other circulatory problems. Since platelets have no DNA, they are unable to synthesize new COX once aspirin has irreversibly inhibited the enzyme, rendering them "useless": an important difference with reversible inhibitors.
Furthermore, aspirin has two additional modes of actions, contributing to its strong analgesic, antipyretic, and anti-inflammatory properties:
- It uncouples oxidative phosphorylation in cartilaginous (and hepatic) mitochondria.
- It induces the formation of NO-radicals in the body that enables the white blood cells (leukocytes) to fight infections more effectively. This has been found recently by Dr. Derek W. Gilroy, winning Bayer's International Aspirin Award 2005.
Also, recently aspirin has been proven to prevent carcinoma of the colon, if given in low doses over years.
Indications
Aspirin, as with many older drugs, has proven to be useful in many conditions. Despite its well-known toxicity, it is widely used, since physicians are familiar with its properties. Indications for its use include:
- Fever
- Pain (especially useful for some forms of arthritis, osteoid osteoma, and chronic pain)
- Migraine
- Rheumatic fever (drug of choice)
- Kawasaki's disease (along with IVIG)
- Pericarditis
In addition, it is recommended (low dose, 75-81 mg daily) for the prevention of:
- Myocardial infarction - in patients with risk factors for cardiovascular disease
- Stroke - as secondary prevention (i.e. to prevent recurrence)
Contraindications and warnings
- Aspirin should be avoided by those known to be allergic to aspirin, ibuprofen, or naproxen.
- It is generally recommended that one seek medical help if symptoms do not improve after a few days of therapy.
- Caution should be taken in patients with kidney disease, peptic ulcers, mild diabetes, gout, or gastritis; manufacturers recommend talking to one's doctor before using this medicine.
- Taking aspirin with alcohol increases the chance of stomach bleeding.
- Children, including teenagers, are discouraged from using aspirin in cold or flu symptoms as this has been linked with Reye's syndrome.
- Patients with hemophilia or other bleeding tendencies should not take salicylates.
- Some sources recommend that patients with hyperthyroidism avoid aspirin because it elevates T4 levels. [2]
Common side effects
- Gastrointestinal complaints (stomach upset, dyspepsia, heartburn, small blood loss). To help avoid these problems, it is recommended that aspirin be taken at or after meals. Undetected blood loss may lead to hypochromic anemia.
- Severe gastrointestinal complaints (gross bleeding and/or ulceration), requiring discontinuation and immediate treatment. Patients receiving high doses and/or long-term treatment should receive gastric protection with high-dosed antacids, ranitidine, or omeprazole.
- Frequently, central nervous system effects (dizziness, tinnitus, hearing loss, vertigo, centrally mediated vision disturbances, and headaches). The higher the daily dose is, the more likely it is that central nervous system side effects will occur.
- Sweating, seen with high doses, independent from antipyretic action
- With long-term treatment with high doses (for arthritis and rheumatic fever), often increased liver enzymes without symptoms, rarely reversible liver damage. The potentially fatal Reye's syndrome may occur, if given to pediatric patients with fever and other signs of infections. The syndrome is due to fatty degeneration of liver cells. Up to 30 percent of those afflicted will eventually die. Prompt hospital treatment may be life-saving.
- Chronic nephritis with long-term use, usually if used in combination with certain other painkillers. This condition may lead to chronic renal failure.
- Prolonged and more severe bleeding after operations and post-traumatic for up to 10 days after the last aspirin dose. If one wishes to counteract the bleeding tendency, fresh thrombocyte concentrate will usually work.
- Skin reactions, angioedema,, and bronchospasm have all been seen infrequently.
Overdose
Aspirin overdose has serious consequences and is potentially lethal. Possible effects of overdose include tinnitus, abdominal pain, hypokalemia, hypoglycemia, pyrexia, hyperventilation, dysrhythmia, hypotension, hallucination, renal failure, confusion, seizure, coma, and death.
Overdose can be acute or chronic; that is, a person can overdose by taking one very large dose or smaller doses over a period of time. Acute overdose has a mortality rate of two percent. Chronic overdose is more commonly lethal with a mortality rate of 25 percent. The most common cause of death during an aspirin overdose is noncardiogenic pulmonary edema.
An acute-overdose patient must be taken to a hospital immediately. Contrary to an urban legend, one can die from eating a bottle of pills, even if they are subsequently thrown up. Treatment of an acute overdose requires ingestion of activated charcoal to neutralize the acetylsalicylic acid in the gastrointestinal tract, followed by a stomach pump with subsequent re-ingestion of activated charcoal. Patients are then monitored for at least 12 hours and typically given intravenous potassium chloride to counteract hypokalemia; sodium bicarbonate to neutralize salicylate in the blood and restore the blood's sensitive pH balance' and glucose to restore blood sugar levels. Frequent blood work is performed to check metabolic, salicylate, and blood sugar levels; arterial blood gas assessments are performed to test for respiratory alkalosis, and metabolic acidosis. If the overdose was intentional, the patient should undergo psychiatric evaluation, as with any suicide attempt.
Fifty-two deaths involving single-ingredient aspirin were reported in the United States in the year 2000 (Litovitz 2001).
ReferencesISBN links support NWE through referral fees
- Litovitz, T. L., W. Klein-Schwartz, S. White, D. J. Cobaugh, J. Youniss, J. c. Omslaer, A. Drab, and B. E. Benson. 2001. 2000 âAnnual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System.â Am J Emerg Med 19 (5): 337-95. PMID 11555795
- McTavish, J. 1987. âWhat's in a name? Aspirin and the American Medical Association.â Bulletin of the History of Medicine 61: 364-365.
- Rainsford, K. D. 1984. Aspirin and the Salicylates. London: Butterworths.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.