Benzene
| Benzene | |
|---|---|
| |
| General | |
| Systematic name | Benzene |
| Other names | Benzol |
| Molecular formula | C6H6 |
| SMILES | c1ccccc1 C1=CC=CC=C1 |
| InChI | InChI=1/C6H6 /c1-2-4-6-5-3-1/h1-6H |
| Molar mass | 78.11 g/mol |
| Appearance | Colorless liquid |
| CAS number | [71-43-2] |
| Properties | |
| Density and phase | 0.8786 g/cmÂł, liquid |
| Solubility in water | 1.79 g/L (25 °C) |
| Melting point | 5.5 °C (278.6 K) |
| Boiling point | 80.1 °C (353.2 K) |
| Viscosity | 0.652 cP at 20 °C |
| Structure | |
| Molecular shape | Planar |
| Symmetry group | D6h |
| Dipole moment | 0 D |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Flammable (F) Carc. Cat. 1 Muta. Cat. 2 Toxic (T) |
| NFPA 704 | |
| R-phrases | R45, R46, R11, R36/38, R48/23/24/25, R65 |
| S-phrases | S53, S45 |
| Flash point | â11 °C |
| Autoignition temperature | 561 °C |
| RTECS number | CY1400000 |
| Related compounds | |
| Related hydrocarbons |
cyclohexane naphthalene |
| Related compounds | toluene borazine |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) | |
Benzene (also known as benzol or [6]-annulene) is a colorless, flammable, sweet-smelling liquid. It is a natural constituent of crude oil but is usually synthesized from other compounds present in petroleum. Chemically, it is classified as an aromatic hydrocarbon, which is a group of organic compounds. Its chemical formula is C6H6. If inhaled or ingested in relatively large amounts, it can cause serious health problems, including cancer, and may even lead to premature death. On the other hand, through years of patient effort, scientists have found that it is a valuable solvent and an important precursor in the production of a wide range of materials, including drugs, plastics, synthetic rubber, and dyes.
History
Benzene has been the subject of studies by many famous scientists, including Michael Faraday and Linus Pauling. In 1825, Faraday reported its isolation from oil gas and gave it the name bicarburet of hydrogen. In 1833, Eilhard Mitscherlich produced it by the distillation of benzoic acid (from gum benzoin) and lime (calcium oxide). Mitscherlich named the compound benzin. In 1845, Charles Mansfield, working under August Wilhelm von Hofmann, isolated benzene from coal tar. Four years later, Mansfield began the first industrial-scale production of benzene, based on the coal-tar method.
Structure
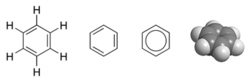
Scientists who were familiar with the chemical formula of benzene (C6H6) were mystified about its molecular structure. They knew that each molecule of benzene contained six carbon atoms, but the substance did not behave as though each molecule was an open-ended chain. Friedrich August KekulĂŠ von Stradonitz is usually credited with being the first to deduce the ring structure of benzene, in 1865. It is reported that after he had spent a long time mentally wrestling over the matter, he had a dream of a snake swallowing its own tail. This image inspired him to think of a ring structure for benzene.
When KekulĂŠ made his formal claims, they were well-publicized and accepted. It now appears that a lesser known scientist, Josef Loschmidt (1821-1895), had posited a cyclic structure for benzene in a booklet published in 1861. Whether KekulĂŠ actually had the dream or whether he borrowed from Loschmidt's publication are matters of debate. It is entirely possible that the two scientists thought of benzene's ring structure independentlyâa type of occurrence that is not unusual in science. The cyclic nature of benzene was finally confirmed by the crystallographer Kathleen Lonsdale.
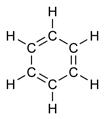
Benzene presents a special problem in that, to account for all the bonds, there must be alternating single and double covalent bonds between carbon atoms, which may be represented as:
Using the technique known as X-ray diffraction, researchers discovered that all the carbon-carbon (C-C) bonds in benzene have the same length (140 picometers (pm)). The length of each C-C bond is greater than that of a double bond (134 pm) but shorter than a single bond (147 pm). The bond length of 140 pm, which is intermediate in length, is explained by the concept of "electron delocalization": the electrons for C-C bonding are distributed equally among the six carbon atoms. (One representation is that the structure exists as a superposition of two "resonance structures," rather than either form individually.)
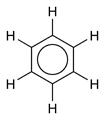
This delocalization of electrons is known as aromaticity, which gives benzene great stability. This enhanced stability is a fundamental property of a class of molecules called "aromatic molecules," differentiating them from molecules that are not aromatic. To reflect the delocalized nature of the bonding, benzene is often depicted with a circle inside a hexagonal arrangement of carbon atoms (which are not labeled):
Substituted benzene derivatives
Many important chemicals are derived from benzene, wherein one or more hydrogen atoms are replaced with other functional groups. Examples of simple benzene derivatives are phenol (C6H5OH, or PhOH), toluene (C6H5CH3, or PhMe), and aniline ((C6H5NH2, or PhNH2). The linking of two benzene rings gives biphenyl (C6H5-C6H5). When two or more aromatic rings are "fused" together, (that is, when a side of one ring is shared with another), the resultant compounds are called fused aromatic hydrocarbons, such naphthalene (with two fused rings) and anthracene (with three fused rings). The limit of the fusion process is the hydrogen-free material graphite.
Some aromatic compounds are called heterocyclic. In these cases, one or more carbon atoms in the benzene ring are replaced with other elements. The most important heterocyclic derivatives are rings containing nitrogen atoms as well as carbon atoms. Examples of heterocyclic compounds are pyridine (C5H5N) and pyrimidine (C4H4N2). (Two other heterocyclic compounds, pyridazine and pyrazine have the same chemical formula as pyrimidine, but the relative positions of the two N atoms in each ring are different.)
Production
Trace amounts of benzene may result whenever carbon-rich materials undergo incomplete combustion. It is produced in volcanoes and forest fires, and is also a component of cigarette smoke.
Up until World War II, benzene was produced mainly as a byproduct of coke production in the steel industry. In the 1950s, however, as the demand for benzene increased, especially from the growing plastics industry, necessitating its production from petroleum. Today, most benzene comes from the petrochemical industry, with only a small fraction being produced from coal.
The industrial production of benzene relies on three major chemical processes: catalytic reforming, toluene hydrodealkylation, and steam cracking. Another process, called toluene disproportionation, may be used when the goal is to produce aromatics called xylenes (there are three types of xylenes) along with benzene.
Catalytic reforming
In catalytic reforming, a mixture of hydrocarbons with boiling points between 60-200 °C is blended with hydrogen gas, exposed to a catalyst (such as platinum chloride or rhenium chloride), and heated at 500-525 °C at pressures between 8 and 50 atmospheres (atm). Under these conditions, aliphatic hydrocarbons form rings and lose hydrogen to become aromatic hydrocarbons. The aromatic products are extracted from the reaction mixture with any of a number of solvents, such as diethylene glycol or sulfolane, and benzene is separated from the other aromatics by distillation.
Toluene hydrodealkylation
In the method known as toluene hydrodealkylation, toluene (C6H5CH3) is converted to benzene. Toluene is mixed with hydrogen, then passed over a catalyst (of chromium, molybdenum, or platinum oxide), at 500-600 °C and 40-60 atm pressure. Sometimes, higher temperatures are used instead of a catalyst. Under these conditions, toluene undergoes dealkylation according to the chemical equation:
The typical reaction yield exceeds 95 percent. Sometimes, xylene and heavier aromatics are used in place of toluene, with similar efficiency.
Toluene disproportionation
If benzene and xylenes are needed, then the method known as toluene disproportionation (TDP) may be an attractive alternative. During the reaction, some toluene molecules lose their methyl groups to produce benzene molecules (as above), while other toluene molecules gain methyl groups to produce xylene molecules (each of which has two methyl groups).
Steam cracking
Steam cracking is the process for producing ethylene and other olefins ("unsaturated hydrocarbons") from aliphatic hydrocarbons. Depending on the feedstock used to produce the olefins, steam cracking can produce a benzene-rich liquid byproduct called pyrolysis gasoline. The latter may be blended with other hydrocarbons as a gasoline additive, or distilled to separate it into its components, including benzene.
Uses
Early uses
In the nineteenth and early twentieth centuries, benzene was used as an after-shave lotion because of its pleasant smell. In addition, prior to the 1920s, benzene was frequently used as an industrial solvent, especially for degreasing metal. As its toxicity became obvious, benzene has been supplanted by other solvents.
In 1903, Lugwig Roselius popularized the use of benzene to decaffeinate coffee. This discovery led to the production of Sanka (-ka for kaffein). This process was later discontinued.
As a gasoline additive, benzene increases the octane rating and reduces engine knock. Consequently, before the 1950s, gasoline often contained several percent benzene. Thereafter, tetraethyl lead replaced benzene as the most widely used antiknock additive. With the global phaseout of leaded gasoline, benzene has made a comeback as a gasoline additive in some countries. In the United States, concern over the negative health effects of benzene and the possibility of it entering groundwater have led to stringent regulations regarding the benzene content of gasolineâthe values are now typically around one percent. European petrol (gasoline) specifications now contain the same one percent limit on benzene content.
Current uses of benzene
Today, benzene is mainly used as an intermediate to make a variety of other chemicals.
- The most widely produced derivatives of benzene include:
- Smaller amounts of benzene are used to make some types of rubbers, lubricants, dyes, detergents, drugs, explosives, and pesticides.
- In laboratory research, toluene is now often used as a substitute for benzene. The solvent properties of the two are similar, but toluene is less toxic and has a wider liquid range.
Reactions of benzene
Benzene can participate in several types of reactions, some of which are given below.
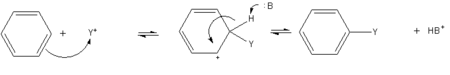
- A general method of derivatizing benzene is known as "electrophilic aromatic substitution." The benzene ring attracts cations, so that hydrogen atoms on the ring can be replaced by acyl or alkyl group to produce substituted derivatives. A generalized reaction is shown on the right, where "Y+" is the cation form of the acyl or alkyl group, and "B" is a base that eventually extracts H+ from the ring.
- A method called Friedel-Crafts acylation is a specific example of electrophilic aromatic substitution. In this reaction, an "acyl" group replaces a hydrogen atom on the benzene ring. The reaction is carried out in the presence of a catalyst such as aluminum chloride.
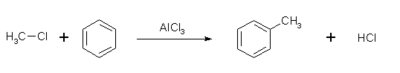
- Likewise, in the method called Friedel-Crafts alkylation, an "alkyl" group replaces a hydrogen atom on the benzene ring (or other aromatic ring). The reaction is carried out with an alkyl halide in the presence of a catalyst.
- Nitration: When benzene is warmed with a combination of concentrated sulphuric and nitric acid, the reaction produces nitrobenzene (C6H5NO2). This reaction, called nitration, is another example of "electrophilic aromatic substitution." It involves the action of "nitronium" ions (NO2+).
- Hydrogenation: Benzene and its derivatives can be converted to cyclohexane (C6H12) and its derivatives by treatment with hydrogen at high pressures. The reaction, called hydrogenation, involves the addition of hydrogen atoms to the aromatic ring.
- Benzene is an excellent ligand in the organometallic chemistry of some metals. Important examples include the sandwich complex, Cr(C6H6)2, and half-sandwich complex, [RuCl2(C6H6)]2.
Health effects
Benzene exposure has serious health effects. Breathing high levels of the substance can result in death, while low levels can cause drowsiness, dizziness, rapid heart rate, headaches, tremors, confusion, and unconsciousness. Eating or drinking foods containing high levels of benzene can cause vomiting, stomach irritation, dizziness, sleepiness, convulsions, rapid heart rate, and death.
The major effect of benzene from chronic (long-term) exposure is on the blood. Benzene damages the bone marrow and can cause a decrease in the production of red blood cells, leading to anemia. It can also cause excessive bleeding and may depress the immune system, increasing the chances of infection.
It has been reported that some women who breathed high levels of benzene for many months had irregular menstrual periods and a decrease in the size of their ovaries. It is not known whether benzene exposure affects the developing fetus in pregnant women or fertility in men.
Animal studies have shown low birth weights, delayed bone formation, and bone marrow damage when pregnant animals breathed benzene.
The U.S. Department of Health and Human Services (DHHS) classifies benzene as a human carcinogen. Long-term exposure to high levels of benzene in the air can cause leukemia, a potentially fatal cancer of the blood-forming organs. In particular, acute myeloid leukemia (AML) may be caused by benzene.
There are several tests to indicate whether a person has been exposed to benzene. One of these is a breath test, which must be done shortly after exposure. Another test measures benzene in the blood; but because benzene disappears rapidly from the blood, measurements are accurate only for recent exposures.
Once benzene enters the digestive system, it is metabolized, and certain metabolites can be measured in the urine. This test, like the others, must be done shortly after exposure. It is not a reliable indicator of how much benzene a person has been exposed to, because the same metabolites in urine may be derived from other sources.
The U.S. Environmental Protection Agency (EPA) has set the maximum permissible level of benzene in drinking water at 0.005 milligrams per liter (0.005 mg/L). EPA requires that spills or accidental releases into the environment of 10 pounds (4.5 kg) or more of benzene should be reported to the agency.
The U.S. Occupational Safety and Health Administration (OSHA) has set a permissible exposure limit of 1 part of benzene per million parts of air (1 ppm) in the workplace during an 8-hour workday, 40-hour workweek.
In March 2006, the official Food Standards Agency in Britain conducted a survey of 150 brands of soft drinks. It found that four contained benzene at levels higher than the limits specified by the World Health Organization. The affected batches were removed from sale.
In recent history, there have been many examples of the harmful health effects of benzene and its derivatives. For instance, in 1981 in Madrid, people who had ingested benzene-contaminated olive oil suffered from toxic oil syndrome, which caused localized immune suppression. In addition, chronic fatigue syndrome has been highly correlated with people who eat "denatured" food that has been treated with solvents to remove fat, or food that contains benzoic acid.
Workers in various industries that make or use benzene may be at risk of exposure to high levels of this carcinogenic chemical. Industries that involve the use of benzene include rubber manufacturers, oil refineries, chemical plants, shoe manufacturers, and gasoline-related industries. In 1987, OSHA estimated that about 237,000 workers in the United States were potentially exposed to benzene, and it is not known if this number has substantially changed since then.
Water and soil contamination are important pathways for the transmission of benzene. In the United States alone, there are approximately 100,000 different sites that have benzene contamination in the soil or groundwater. In 2005, after an explosion at the China National Petroleum Corporation (CNPC) factory in Jilin, China, benzene leaked into the Songhua River. As the river supplies water to the city of Harbin, with a population of almost nine million, water supply to the city was cut off.
See also
ReferencesISBN links support NWE through referral fees
- Couper, Archibald Scott. 1858. On a New Chemical Theory. Philosophical Magazine 16:104-116.
- Lonsdale, Kathleen. 1929. The Structure of the Benzene Ring in Hexamethylbenzene. Proceedings of the Royal Society 123A:494.
- Lonsdale, Kathleen. An X-Ray Analysis of the Structure of Hexachlorobenzene, Using the Fourier Method. Proceedings of the Royal Society 133A:536.
- McMurry, John. 2004. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0136436692.
- Solomons, T.W. Graham, and Fryhle, Craig B. 2004. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
- Associated Press. May 19, 2006. FDA: Too Much Benzene In Some Drinks. CBS News.
External links
All links retrieved September 28, 2023.
- NIOSH Pocket Guide to Chemical Hazards
- Dept. of Health and Human Services: TR-289: Toxicology and Carcinogenesis Studies of Benzene
| Functional groups |
|---|
| Chemical class: Alcohol ⢠Aldehyde ⢠Alkane ⢠Alkene ⢠Alkyne ⢠Amide ⢠Amine ⢠Azo compound ⢠Benzene derivative ⢠Carboxylic acid ⢠Cyanate ⢠Ester ⢠Ether ⢠Haloalkane ⢠Imine ⢠Isocyanide ⢠Isocyanate ⢠Ketone ⢠Nitrile ⢠Nitro compound ⢠Nitroso compound ⢠Peroxide ⢠Phosphoric acid ⢠Pyridine derivative ⢠Sulfone ⢠Sulfonic acid ⢠Sulfoxide ⢠Thioether ⢠Thiol ⢠Toluene derivative |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.