Benzoic acid
| Benzoic acid | |
|---|---|
| |
| |
| IUPAC name | Benzoic acid, benzene carboxylic acid |
| Other names | Carboxybenzene, E210, dracylic acid |
| Identifiers | |
| CAS number | [] |
| PubChem | |
| KEGG | |
| MeSH | |
| ChEBI | |
| RTECS number | DG0875000 |
| SMILES | c1ccccc1C(=O)O |
| InChI | InChI=1/C7H6O2/c8-7(9)6-4-2-1-3-5-6/ h1-5H,(H,8,9)/f/h8H |
| Beilstein Reference | 636131 |
| Gmelin Reference | 2946 |
| 3DMet | |
| Properties | |
| Molecular formula | C6H5COOH |
| Molar mass | 122.12 g/mol |
| Appearance | Colourless crystalline solid |
| Density | 1.32 g/cm3, solid |
| Melting point |
122.4 °C (395 K) |
| Boiling point |
249 °C (522 K) |
| Solubility in water | Soluble (hot water) 3.4 g/l (25 °C) |
| Solubility in methanol, diethylether | Soluble |
| Acidity (pKa) | 4.21 |
| Structure | |
| Crystal structure | Monoclinic |
| Molecular shape | planar |
| Dipole moment | 1.72 D in Dioxane |
| Hazards | |
| MSDS | ScienceLab.com |
| Main hazards | Irritating |
| NFPA 704 |
|
| R-phrases | R22, R36 |
| S-phrases | S24 |
| Flash point | 121 °C (394 K) |
| Related Compounds | |
| Related carboxylic acid | phenylacetic acid, hippuric acid, salicylic acid |
| Related compounds | benzene, benzaldehyde, benzyl alcohol, benzylamine, benzyl benzoate, benzoyl chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Benzoic acid, C7H6O2 (or C6H5COOH), is a colorless crystalline solid and the simplest aromatic carboxylic acid. The name derived from gum benzoin, which was for a long time the only source for benzoic acid. This weak acid and its salts are used as a food preservative. Benzoic acid is an important precursor for the synthesis of many other organic substances.
History
Benzoic acid was discovered in the 16th century. The dry distillation of gum benzoin was first described by Nostradamus (1556), and subsequently by Alexius Pedemontanus (1560) and Blaise de Vigenère (1596).[1]
Justus von Liebig and Friedrich Wöhler determined the structure of benzoic acid in 1832.[2] They also investigated how hippuric acid is related to benzoic acid.
In 1875 Salkowski discovered the antifungal abilities of benzoic acid, which were used for a long time in the preservation of benzoate containing fruits.[3]
Production
Industrial preparations
Benzoic acid is produced commercially by partial oxidation of toluene with oxygen. The process is catalyzed by cobalt or manganese naphthenates. The process uses cheap raw materials, proceeds in high yield, and is considered environmentally attractive.
U.S. production capacity is estimated to be 126 000 tonnes per year, much of which is consumed domestically to prepare other industrial chemicals.
Historical preparations
The first industrial process involved the reaction of benzotrichloride (trichloromethyl benzene) with calcium hydroxide in water, using iron or iron salts as catalyst. The resulting calcium benzoate is converted to benzoic acid with hydrochloric acid. The product contains significant amounts of chlorinated benzoic acid derivatives. For this reason, benzoic acid for human consumption was obtained by dry distillation of gum benzoin. Even after the discovery of other synthesis methods, it was forbidden to use benzoic acid of other source than gum benzoin.
Alkyl substituted benzene derivatives give benzoic acid with the stoichiometric oxidants potassium permanganate, chromium trioxide, nitric acid.
Uses
Food preservative
Benzoic acid and its salts are used as a food preservative, represented by the E-numbers E210, E211, E212, and E213. Benzoic acid inhibits the growth of mold, yeast[4] and some bacteria. It is either added directly or it is created from reactions with its sodium, potassium or calcium salt. The mechanism starts with the absorption of benzoic acid in to the cell. If the intracellular pH changes to 5 or lower the anaerobic fermentation of glucose through phosphofructokinase is decreased by 95 percent. The effectivity of benzoic acid and benzoate is thus dependent on the pH of the food.[5] Acidic food and beverage like fruit juice (citric acid), sparkling drinks (carbon dioxide), soft drinks (phosphoric acid), pickles (vinegar) or other acidified food are preserved with benzoic acid and benzoates.
Concern has been expressed that benzoic acid and its salts may react with ascorbic acid (vitamin C) in some soft drinks, forming small quantities of benzene. [6]
Synthesis of other chemicals
Benzoic acid is used to make a large number of chemicals, important examples:
- Benzoyl chloride, C6H5C(O)Cl, is obtained by treatment of benzoic with thionyl chloride, phosgene or one of the chlorides of phosphorus. C6H5C(O)Cl is an important starting material for several benzoic acid derivates like benzyl benzoate, which is used as artificial flavours and insect repellents.
- Benzoyl peroxide, [C6H5C(O)O]2, is obtained by treatment with peroxide.[7] The peroxide is a radical starter in polymerization reactions and also a component in cosmetic products.
- Benzoate plasticizers, such as the glycol-, diethylengylcol-, and triethyleneglycol esters are obtained by transesterification of methyl benzoate with the corresponding diol. Alternatively these species arise by treatment of benzoylchloride with the diol. These plasticizers are used similarly to those derived from terephthalic acid ester.
- Phenol, C6H5OH, is obtained by oxidative decarboxylation at 300-400°C. The temperature required can be lowered to 200°C by the addition of catalytic amounts of copper(II) salts. The phenol can be converted to cyclohexanol, which is than starting material for nylon synthesis.
Medicinal
Benzoic acid is a constituent of Whitfield Ointment which is used for the treatment of fungal skin diseases such as tinea, ringworm and athlete's foot. It is also considered an effective treatment for acne.
Purification
Benzoic acid is purified by a method called recrystallisation. Process starts with crystalline material, removes all the impurities and forms new crystals.
Biology and health effects
Gum benzoin contains up to 20 percent of benzoic acid and 40 percent benzoic acid esters.[8]
Benzoic acid is present as part of hippuric acid (N-Benzoylglycine) in urine of mammals, especially herbivores (Gr. hippos = horse; ouron = urine). Humans produce about 0.44 g/L hippuric acid per day in their urine, and if the person is exposed to toluene or benzoic acid it can rise above that level.[9]
For humans the WHO's International Programme on Chemical Safety (IPCS) suggests a provisional tolerable intake would be 5 mg/kg body weight per day.[10] Cats have a significantly lower tolerance against benzoic acid and its salts than rats and mice. Lethal dose for cats can be as low as 300 mg/kg body weight.[11] The oral LD50 for rats is 3040 mg/kg, for mice it is 1940-2263 mg/kg.[12]
Chemistry
Reactions of benzoic acid can occur at either the aromatic ring or the carboxylic group:
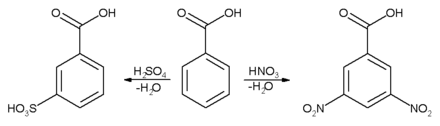
Aromatic ring
Electrophilic aromatic substitution reaction will take place mainly in 3-position to the electron-withdrawing carboxylic group.
The second substitution reaction (on the right) is slower because the first nitro group is deactivating.[13] Conversely, if an activating group (electron-donating) was introduced (eg alkyl), a second substitution reaction would occur more readily than the first and the disubstituted product might not accumulate to a significant extent.
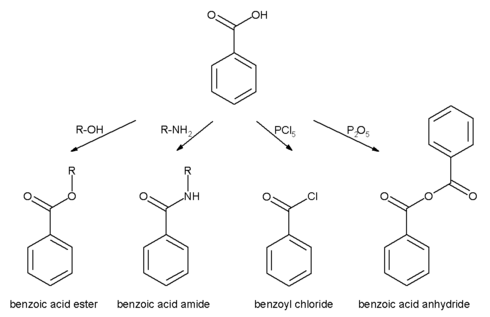
Carboxylic group
All the reactions mentioned for carboxylic acids are also possible for benzoic acid.
- Benzoic acid esters are the product of the acid catalysed reaction with alcohols.
- Benzoic acid amides are more easily available by using activated acid derivatives (such as benzoyl chloride) or by coupling reagents used in peptide synthesis like DCC and DMAP.
- The more active benzoic anhydride is formed by dehydration using acetic anhydride or phosphorus pentoxide.
- Highly reactive acid derivatives such as acid halides are easily obtained by mixing with halogenation agents like phosphorus chlorides or thionyl chloride.
- Orthoesters can be obtained by the reaction of alcohols under acidic water free conditions with benzonitrile.
- Reduction to benzaldehyde and benzyl alcohol is possible using DIBAL-H, LiAlH4 or sodium borohydride.
- The copper catalysed decarboxylation of benzoate to benzene may be effected by heating in quinoline. Alternatively, Hunsdiecker decoarboxylation can be achieved by forming the silver salt and heating.
Laboratory preparations
Benzoic acid is cheap and readily available, so the laboratory synthesis of benzoic acid is mainly practiced for its pedogical value. It is a common undergraduate preparation and an unusual feature of the compound is that its melting point equals its molecular weight (122). For all syntheses, benzoic acid can be purified by recrystallization from water owing to its high solubility in hot and poor solubility in cold water. The avoidance of organic solvents for the recrystallization makes this experiment particularly safe.
By hydrolysis
Like any other nitrile or amide, benzonitrile and benzamide can be hydrolyzed to benzoic acid or its conjugate base in acid or basic conditions.
From benzaldehyde
The base-induced disproportionation of benzaldehyde, the Cannizzaro reaction, affords equal amounts of benzoate and benzyl alcohol; the latter can be removed by distillation.
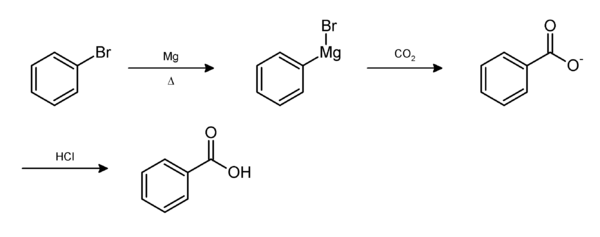
From bromobenzene
Bromobenzene in diethyl ether is stirred with magnesium turnings to produce phenylmagnesium bromide (C6H5MgBr). This Grignard reagent is slowly added to dry ice (solid carbon dioxide) to give benzoate. Dilute acid is added to form benzoic acid.
From benzyl alcohol
Benzyl alcohol is refluxed with potassium permanganate or other oxidizing reagents in water. The mixture hot filtered to remove manganese oxide and then allowed to cool to afford benzoic acid.
Notes
- ↑ O-A Neumüller. 1988. Römpps Chemie-Lexikon, edition 6. (Stuttgart: Frankh'sche Verlagshandlung. ISBN 3440045161)
- ↑ Liebig J, Wöhler F Untersuchungen über das Radikal der Benzoesäure. Liebigs Annalen|Annalen der Chemie (1832) 3: 249-282 10.1002/jlac.18320030302
- ↑ E. Salkowski. Berl Klin Wochenschr (1875) 12:297-298
- ↑ A. D. Warth. Mechanism of action of benzoic acid on Zygosaccharomyces bailii: effects on glycolytic metabolite levels, energy production, and intracellular pH. Appl Environ Microbiol. 1:1(December 1991) [1]
- ↑ I. Pastrorova, C.G. de Koster, J.J. Boom. Analytic Study of Free and Ester Bound Benzoic and Cinnamic Acids of Gum Benzoin Resins by GC-MS HPLC-frit FAB-MS. Phytochem Anal 8 (1997): 63-73 doi 10.1002/(SICI)1099-1565(199703)8:2<63::AID-PCA337>3.0.CO;2-Y
- ↑ Indications of the possible formation of benzene from benzoic acid in foods[2] BfR article, Dec. 1, 2005.
- ↑ L. S. Silbert, E. Siegel, D. Swern, "Peroxybenzoic Acid" Organic Syntheses, (Collected Volume 5,(1973): 904
- ↑ K. Tomokuni, M. Ogata. Direct Colorimetric Determination of Hippuric Acid in Urine. Clin Chem 18 (1972): 349-351
- ↑ H.A. Krebs, D. Wiggins, M. Stubbs. Studies on the mechanism of the antifungal action of benzoate. Biochem J 214 (1983): 657-663 [3]
- ↑ Concise International Chemical Assessment Document 26: BENZOIC ACID AND SODIUM BENZOATE
- ↑ P.G. Bedford, E.G. Clarke. Experimental benzoic acid poisoning in the cat. Vet Rec 90 (1972):53-58 PMID 4672555
- ↑ >Concise International Chemical Assessment Document 26: BENZOIC ACID AND SODIUM BENZOATE
- ↑ Brewster, R. Q.; Williams, B.; Phillips, R. 3,5-Dinitrobenzoic Acid. Organic Syntheses, Collected Volume 3(1955): 337
ReferencesISBN links support NWE through referral fees
- McMurry, John. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole, 2004. ISBN 0534420052
- Morrison, Robert T., and Robert N. Boyd. Organic Chemistry, 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0136436692
- Solomons, T.W. Graham, and Craig B. Fryhle. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998
- Cosmetic Ingredient Review Expert Panel, Bindu Nair. Final Report on the Safety Assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. Int. J. Tox. 20(Suppl. 3) (2001): 23-50.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.