Citric acid
| Citric acid | |
|---|---|
| |
| General | |
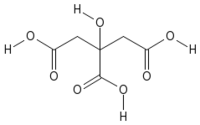
| Systematic name | 2-hydroxypropane- 1,2,3-tricarboxylic acid |
| Other names | ? |
| Empirical formula | C6H8O7 |
| SMILES | C(C(=O)O)C(CC(=O)O)(C(=O)O)O |
| Molar mass | 192.027 g/mol |
| Appearance | crystalline white solid |
| CAS number | [77-92-9] |
| Properties | |
| Density and phase | 1.665 g/cm³ |
| Solubility in water | 133 g/100 ml (20°C) |
| Melting point | 153 °C (307.4 °F, 426 K) |
| Boiling point | decomposes at 175 °C (448 K) |
| Acidity (pKa) | pKa1=3.15 pKa2=4.77 pKa3=6.40 |
| Viscosity | ? cP at ?°C |
| Structure | |
| Crystal structure | ? |
| Dipole moment | ? D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | skin and eye irritant |
| NFPA 704 | |
| Flash point | ?°C |
| R/S statement | R: ? S: ? |
| RTECS number | ? |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related compounds | sodium citrate, calcium citrate |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references | |
Citric acid is a weak organic acid found in citrus fruits, which are fruits of flowering plants of the genus Citrus in the family Rutaceae, originating in tropical and subtropical Southeast Asia, and including lemon, grapefruit, orange, tangerine, and lime. In biochemistry, citric acid is important as an intermediate in the citric acid cycle and therefore occurs in the metabolism of almost all living things.
Citric acid shows both the good and bad use of human creativity. Production techniques have been developed for its uses as a food additive—citric acid is a natural preservative and is also used to add an acidic (sour) taste to foods and soft drinks—;as an environmentally benign cleaning agent; as an antioxidant (slows or prevents the oxidation of other chemicals); to keep fat globules separate in ice cream; among many uses. On the other hand, citric acid is required to produce HMTD, an explosive with concern in terms of terrorism, and as a buffer to increase the solubility of brown heroin in the illegal drug industry.
Worldwide, about one million tons of citric acid are commercially produced each year (Soccol et al., 2003).
Citric acid exists in a variety of fruits and vegetables, but it is most concentrated in lemons and limes, where it can comprise as much as eight percent of the dry weight of the fruit.
Properties
At room temperature, citric acid is a white crystalline powder. It can exist either in an anhydrous (water-free) form, or as a monohydrate that contains one water molecule for every molecule of citric acid. The anhydrous form crystallizes from hot water, while the monohydrate forms when citric acid is crystallized from cold water. The monohydrate can be converted to the anhydrous form by heating it above 74°C.
Citric acid also dissolves in absolute (anhydrous) ethanol (76 parts of citric acid per 100 parts of ethanol) at 15°C.
Chemically, citric acid shares the properties of other carboxylic acids. (organic acids characterized by the presence of a carboxyl group, which has the formula -C(=O)OH, usually written -COOH or -CO2H.) When heated above 175 \°C, it decomposes through the loss of carbon dioxide and water.
History of Discovery
The discovery of citric acid has been credited to the eighth-century alchemist Jabir Ibn Hayyan (also known as Geber), who also discovered hydrochloric acid (from salt), nitric acid (from saltpeter), acetic acid (from vinegar), and tartaric acid (from wine-making residues).
Medieval scholars in Europe were aware of the acidic nature of lemon and lime juices; such knowledge is recorded in the thirteenth-century encyclopedia Speculum Majus (The Great Mirror), compiled by Vincent of Beauvais.
Citric acid was first isolated in 1784 by the Swedish chemist Carl Wilhelm Scheele, who crystallized it from lemon juice.
Production
Industrial-scale citric acid production began in 1860, based on the Italian citrus fruit industry.
In 1893, C. Wehmer discovered that Penicillium mold could produce citric acid from sugar. However, microbial production of citric acid did not become industrially important until World War I disrupted Italian citrus exports. In 1917, the American food chemist James Currie discovered that certain strains of the mold Aspergillus niger could be efficient citric acid producers, and Pfizer began industrial-level production using this technique two years later.
In this production technique, which is still the major industrial route to citric acid used today, cultures of Aspergillus niger are fed on sucrose to produce citric acid. After the mold is filtered out of the resulting solution, citric acid is isolated by precipitating it with lime (calcium hydroxide) to yield calcium citrate salt, from which citric acid is regenerated by treatment with sulfuric acid.
Alternatively, citric acid is sometimes isolated from the fermentation broth by liquid-liquid extraction with a hydrocarbon solution of the organic base trilaurylamine, followed by re-extraction from the organic solution by water.
Annual production of citric acid is about one million tons, making citric acid one of the fermentation products with the highest levels of production worldwide (Soccol et al., 2003). About 70% of the total citric acid production is consumed by the food industry (Prado et al. 2005).
Citric Acid Cycle
Citric acid is one of a series of compounds involved in metabolic pathway that forms part of the breakdown of carbohydrates, fats, and proteins into carbon dioxide and water in order to generate energy. This is the citric acid cycle (also known as the tricarboxylic acid cycle and as the Krebs cycle).
The citric acid cycle is a series of chemical reactions of central importance in all living cells that utilize oxygen to generate useful energy by cellular respiration. Essentially, the cycle involves converting the potential energy of a variety of nutrients into the readily available energy of adenosine triphosphate (ATP). This cycle is the "power plant" that energizes all metabolism.
Citrate is an intermediary in the citric acid cycle. A citrate is an ionic form of citric acid, such as C3H5O(COO)33−, that is, citric acid minus three hydrogen ions.
The citric acid cycle the source of two-thirds of the food-derived energy in higher organisms.
Uses
As a food additive, citric acid is used as a flavoring and preservative in food and beverages, especially soft drinks. It is denoted by E number E330. (E numbers are codes for food additives used on food labels in the European Union and some countries outside this region.) Citrate salts of various metals are used to deliver those minerals in a biologically available form in many dietary supplements. The buffering properties of citrates are used to control pH in household cleaners and pharmaceuticals.
Citric acid's ability to chelate metals (reversible binding of a ligant to a metal ion) makes it useful in soaps and laundry detergents. By chelating the metals in hard water, it lets these cleaners produce foam and work better without need for water softening. Similarly, citric acid is used to regenerate the ion exchange materials used in water softeners by stripping off the accumulated metal ions as citrate complexes.
Citric acid is the active ingredient in some bathroom and kitchen cleaning solutions. A solution with a six percent concentration of citric acid will remove hard water stains from glass without scrubbing.
Citric acid is used in the biotechnology and pharmaceutical industry to passivate (make material passive or non-reactive) high purity process piping (in lieu of using nitric acid). Nitric acid is considered hazardous to dispose once used for this purpose, while citric acid is not.
Citric acid can be added to ice cream to keep fat globules separate and can be added to recipes in place of fresh lemon juice as well. Citric acid is used along with sodium bicarbonate in a wide range of effervescent formulas, both for ingestion (e.g., powders and tablets) and for personal care (e.g., bath salts, bath beads, and cleaning of grease).
When applied to hair, citric acid opens up the outer layer, also known as the cuticle. While the cuticle is open, it allows for a deeper penetration into the hair shaft. It can be used in shampoo to wash out wax and coloring from the hair. It is notably used in the product "Sun-in" for bleaching, but is generally not recommended due to the amount of damage it causes.
Citric acid is also used as a stop bath in photography. The developer is normally alkaline, so a mild acid will neutralize it, increasing the effectiveness of the stop bath when compared to plain water.
Citric acid is one of the chemicals required for the synthesis of hexamethylene triperoxide diamine (HMTD), a highly heat-, friction-, and shock-sensitive explosive similar to acetone peroxide. Purchases of large quantities of citric acid may rouse suspicion of potential terrorist activity.
Safety
Citric acid is recognized as safe for use in food by all major national and international food regulatory agencies. It is naturally present in almost all forms of life, and excess citric acid is readily metabolized and eliminated from the body.
Interestingly, despite its ubiquity, intolerance to citric acid in the diet is known to exist. Little information is available as the condition appears to be rare, but like other types of food intolerance it is often described as a "pseudo-allergic" reaction.
Contact with dry citric acid or with concentrated solutions can result in skin and eye irritation, so protective clothing should be worn when handling these materials.
There have been erroneous reports that E330 is a major cause of cancer. It is thought that this has been brought about by misunderstanding and confusion over the word Krebs. In this case, it refers to Sir Hans Adolf Krebs, discoverer of the Krebs cycle, and not the German word for cancer. Citric acid is not known to be harmful to the body when taken alone.
ReferencesISBN links support NWE through referral fees
- Coastal Scents. 2007. Citric Acid MSDS. Coastal Scents. Retrieved March 15, 2007.
- Garden, J., K. Roberts, A. Taylor, and D. Robinson. 2003. Evaluation of the Provision of Single Use Citric Acid Sachets to Injecting Drug Users. Scottish Center for Infection and Environmental Health. Retrieved March 15, 2007.
- Prado, F. C., L. P. S. Vandenberghe, A. L. Woiciechowski, J. A. Rodrígues-León, and C. R. Socco. 2005. Citric acid Production by Solid-State Fermentation on a Semi-Pilot Scale Using Different Percentages of Treated Cassava Bagasse. Brazilian Journal of Chemical Engineering 22(4). Retrieved March 15, 2007.
- Soccol, C. R., F. C. Prado, L. P. S. Vandenberghe, and A. Pandey (ed.). 2003. "General Aspects in Citric Acid Production by Submerged and Solid-State Fermentation." In Concise Encyclopedia of Bioresource Technology, edited by A. Pandey, 652-664. New York: Haworth Press. ISBN 1560229802.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.

