Butane
| n-Butane | |
|---|---|

| |
| Identifiers | |
| CAS number | [] |
| SMILES | CCCC |
| Properties | |
| Molecular formula | C4H10 |
| Molar mass | 58.124 g/mol |
| Appearance | Colorless gas |
| Density | 2.48 g/l, gas (15 °C, 1 atm) |
| Melting point |
−138.4 °C (135.4 K) |
| Boiling point |
−0.5 °C (272.6 K) |
| Solubility in water | 6.1 mg/100 ml (20 °C) |
| Hazards | |
| EU classification | Highly flammable (F+) |
| NFPA 704 |
|
| Flash point | −60 °C |
| Related Compounds | |
| Related alkanes | Propane; Pentane |
| Related compounds | Isobutane; Cyclobutane |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Butane, also called n-butane (normal butane), is an unbranched alkane with four carbon atoms in each molecule. Its molecular formula may be written as CH3CH2CH2CH3. Butane is also used as a collective term for both n-butane and its only other isomer, isobutane (also called i-butane or methylpropane), CH(CH3)3. Both isomers of butane are highly flammable, colorless gases at ordinary temperatures and atmospheric pressure. They can be extracted from natural gas or obtained by refining petroleum.
Butane is useful for many applications. Bottled butane gas is a fuel for cooking and camping. It is also used as a component of gasoline (petrol), as a feedstock component for the production of base petrochemicals in steam cracking, and as a fuel for cigarette lighters. When butane is blended with propane and other hydrocarbons, the mixture is referred to commercially as liquefied petroleum gas (LPG), which is a fuel for heating appliances and vehicles.
Recent concerns regarding depletion of the ozone layer by freon gases (chlorofluorocarbons or halomethanes) have led to increased use of isobutane and LPG as refrigerants, especially in domestic refrigerators and freezers, and as propellants in aerosol sprays.
| Isobutane | |
|---|---|

|

|

| |
| IUPAC name | Isobutane Methylpropane |
| Other names | 2-Methylpropane |
| Identifiers | |
| CAS number | [] |
| SMILES | C(C)CC |
| Properties | |
| Molecular formula | C4H10 |
| Molar mass | 58.12 g mol-1 |
| Appearance | colorless gas |
| Density | 2.51 g/l, gas (15 °C, 1 atm);593.4 kg · m-3, liquid |
| Melting point |
-159.6 °C, 114 K, -255 °F |
| Boiling point |
-11.7 °C, 261 K, 11 °F |
| Solubility in water | Insoluble |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Highly flammable (F+) |
| NFPA 704 |
|
| R-phrases | R12 |
| S-phrases | S2, S9, S16 |
| Flash point | flammable gas |
| Autoignition temperature |
460 °C |
| Explosive limits | 1.8–8.4 percent |
| Related Compounds | |
| Related alkane | Butane |
| Related compounds | Isopentane Neopentane |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Etymology
The name butane was derived by back-formation from the name butyric acid. The latter is a carboxylic acid with four carbon atoms, including the carbon atom in the carboxyl group. The molecular formula of butyric acid may be written as CH3CH2CH2COOH.
Properties
At ordinary temperatures and pressures, n-butane is a colorless gas. Its boiling point is −0.5 °C (272.6 K), and its melting point is −138.4 °C (135.4 K). Likewise, i-butane, the structural isomer of n-butane is a colorless gas, with a boiling point of -11.7 °C (261 K) and a melting point of -159.6 °C (114 K). Both isomeric forms can be readily liquefied at room temperature by compression.
Structural isomers
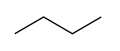
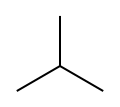
A molecule of either isomer, n-butane or i-butane, is made up of four carbon atoms and ten hydrogen atoms, so their common molecular formula is C4H10. However, the molecular structures of these isomers differ. In particular, a molecule of n-butane consists of a linear arrangement of the four carbon atoms, so that it can also be represented as CH3-CH2-CH2-CH3. By contrast, a molecule of i-butane has three carbon atoms attached directly to a central carbon atom. The structures of these two isomers are shown in the diagrams below.
- Structures of the two isomers of butane
Reactions
When oxygen is plentiful, butane burns to form carbon dioxide and water vapor; when oxygen is limited, carbon (soot) or carbon monoxide may also be formed. The reaction may be written as follows:
- 2C4H10 + 13O2 → 8CO2 + 10H2O
n-Butane is the feedstock for DuPont's catalytic process for the preparation of maleic anhydride (C2H2(CO)2O), as shown by the following reaction:
- CH3CH2CH2CH3 + 3.5O2 → C2H2(CO)2O + 4H2O
n-Butane, like all hydrocarbons, undergoes free radical chlorination producing both 1-chlorobutane and 2-chlorobutane, as well as more highly chlorinated derivatives.

Uses
Butane gas is sold bottled as a fuel for cooking and camping. When blended with propane and other hydrocarbons, it is referred to commercially as Liquified Petroleum Gas (LPG). It is also used as a component of gasoline (petrol), as a feedstock for the production of base petrochemicals in steam cracking, and as a fuel for cigarette lighters.
The highly purified form of isobutane is useful as a refrigerant and as a propellant in aerosol sprays. It is used, for instance, in household refrigerators and freezers, largely replacing the halomethanes that are believed to deplete the ozone layer. When used as a refrigerant or propellant, isobutane may be referred to as R-600a. It is also used as a feedstock in the petrochemical industry, such as for the synthesis of isooctane.[1]
When used as a refrigerant, the flammability of isobutane is not a major issue because the amount of this substance in an appliance is not enough to cause a combustible mix, given the amount of air in a room. The system operating pressure for butane is lower than that for the halomethanes, such as Dichlorodifluoromethane (R-12). Thus, the direct conversion of R-12 systems to butane, such as in automotive air conditioning systems, will not function optimally.
Effects and health issues
Inhaling butane can cause drowsiness, narcosis, asphyxia, cardiac arrhythmia, and frostbite. Inhalation can lead to instant death from asphyxiation. Butane is the most commonly misused volatile solvent in the UK, and was the cause of 52 percent of solvent-related deaths in 2000.[2] If butane is sprayed directly into the throat, the jet of fluid may cool rapidly to –20 °C by expansion, causing prolonged laryngospasm.[3] "Sudden Sniffer's Death syndrome," first described by Bass in 1970,[4] is the most common single cause of solvent-related death, resulting in 55 percent of known fatal cases.
See also
Notes
- ↑ www.chemistry.org, Patent Watch, July 31, 2006. Retrieved May 20, 2008.
- ↑ M.E. Field-Smith, B.K. Butland, J.D. Ramsey, and H.R. Anderson, Trends in Death Associated with Abuse of Volatile Substances 1971-2004, Report 19, Division of Community Health Sciences. Retrieved May 20, 2008.
- ↑ J. Ramsey, H.R. Anderson, K. Bloor, et al. An introduction to the practice, prevalence and chemical toxicology of volatile substance abuse. Hum. Toxicol. 8:261–269.
- ↑ M. Bass, Sudden sniffing death. JAMA 212: 2075–79.
ReferencesISBN links support NWE through referral fees
- McMurry, John. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole, 2004. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. Organic Chemistry, 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0136436692.
- Olah, George A., and Árpád Molnár. Hydrocarbon Chemistry. Hoboken, NJ: Wiley-Interscience, 2003. ISBN 978-0471417828.
- Solomons, T.W. Graham, and Craig B. Fryhle. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998.
- Yaws, Carl L. The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals: Physical Properties for More Than 41,000 Organic and Inorganic Chemical Compounds: Coverage for C1 to C100 Organics and Ac to Zr Inorganics. Houston: Gulf Pub. Co., 2005. ISBN 978-0976511373.
External links
All links retrieved November 23, 2023.
| Alkanes | |||||||||||||||||||||||||||||||
|
methane |
| |
ethane |
| |
propane |
| |
butane |
| |
pentane |
| |
hexane |
|||||||||||||||||||||
|
heptane |
| |
octane |
| |
nonane |
| |
decane |
| |
undecane |
| |
dodecane |
| ||||||||||||||||||||
E numbers
| ||
|---|---|---|
| Colours (E100-199) • Preservatives (E200-299) • Antioxidants & Acidity regulators (E300-399) • Thickeners, stabilisers & emulsifiers (E400-499) • pH regulators & anti-caking agents (E500-599) • Flavour enhancers (E600-699) • Miscellaneous (E900-999) • Additional chemicals (E1100-1599) | ||
| Waxes (E900-909) • Synthetic glazes (E910-919) • Improving agents (E920-929) • Packaging gases (E930-949) • Sweeteners (E950-969) • Foaming agents (E990-999) | ||
| Argon (E938) • Helium (E939) • Dichlorodifluoromethane (E940) • Nitrogen (E941) • Nitrous oxide (E942) • Butane (E943a) • Isobutane (E943b) • Propane (E944) • Oxygen (E948) • Hydrogen (E949) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.