Butyric acid
| Butyric acid | |
|---|---|
| |
| |
| IUPAC name | butyric acid |
| Identifiers | |
| CAS number | [] |
| PubChem | |
| MeSH | |
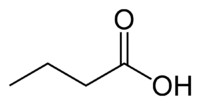
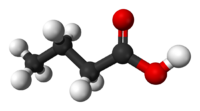
| SMILES | CCCC(=O)O |
| Properties | |
| Molecular formula | C4H8O2 |
| Molar mass | 88.1051 |
| Melting point |
-7.9 °C (265.1 K) |
| Boiling point |
163.5 °C (436.5 K) |
| Hazards | |
| R-phrases | 34 |
| S-phrases | 26 36 45 |
| Flash point | 72 °C |
| RTECS number | ES5425000 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Butyric acid, also known as n-Butanoic acid (in the IUPAC[1] system) or normal butyric acid, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. It is classified as a short-chain fatty acid. It has an unpleasant odor and acrid taste, but a somewhat sweet aftertaste (similar to ether). It is notably found in rancid butter, parmesan cheese, and vomit. Its name is derived from the Greek word βουτυρος, which means "butter." Certain esters of butyric acid have a pleasant taste or smell, and they are used as additives in foods and perfumes.
Occurrence
Normal butyric acid occurs in the form of esters in animal fats and plant oils. Certain bacteria in the mammalian gut transform highly fermentable fibers—such as oat bran, pectin, and guar—into short chain fatty acids, including butyrate.
The glyceride of butyric acid (that is, its ester with glycerol) makes up three to four percent of butter. When butter becomes rancid, butyric acid is released from the glyceride (by a process called hydrolysis), leading to the unpleasant odor.
Normal butyric acid is also found as a hexyl ester in the oil of Heracleum giganteum (cow parsnip) and as an octyl ester in parsnip (Pastinaca sativa). It has also been noticed in the fluids of the flesh and in perspiration.
Preparation
This acid is ordinarily prepared by the fermentation of sugar or starch. The process is carried out by the addition of putrefying cheese, with calcium carbonate added to neutralize the acids formed. The butyric fermentation of starch is aided by the direct addition of Bacillus subtilis.
Notable characteristics
Butyric acid is an oily, colorless liquid that solidifies at -8 °C and boils at 164 °C. It is easily soluble in water, ethanol, and ether, and is thrown out of its aqueous solution by the addition of calcium chloride. The salts and esters of this acid are known as butyrates.
Potassium dichromate and sulfuric acid (or sulphuric acid) oxidize it to carbon dioxide and acetic acid. Alkaline potassium permanganate oxidizes it to carbon dioxide. The calcium salt, Ca(C4H7O2)2•H2O, is less soluble in hot water than in cold.
Butyric acid can be detected by mammals with good scent detection abilities (such as dogs) at ten ppb, while humans can detect it in concentrations above ten ppm.
An isomer, called isobutyric acid, has the same chemical formula (C4H8 O2) but a different structure. It has similar chemical properties but different physical properties.
Applications
Butyric acid is used in the preparation of various butyrate esters. Low-molecular-weight esters of butyric acid, such as methyl butyrate, have mostly pleasant aromas or tastes. As a consequence, they find use as food and perfume additives. They are also used in organic laboratory courses, to teach the Fisher esterification reaction.
Butyrate fermentation
Butyrate is the end-product of a fermentation process performed by obligate anaerobic bacteria. For instance, kombucha tea contains butyric acid as a result of fermentation. This fermentation pathway was discovered by Louis Pasteur in 1861. Examples of butyrate producing bacterial species are:
- Clostridium butyricum
- Clostridium kluyveri
- Clostridium pasteurianum
- Fusobacterium nucleatum
- Butyrivibrio fibrisolvens
- Eubacterium limosum
Butyric acid function/activity in living organisms
Butyrate has diverse effects on cell proliferation, apoptosis (programmed cell death), and differentiation. Different studies have given contrary results in terms of the effect of butyrate on colon cancer. This lack of agreement (particularly between in vivo and in vitro studies) has been termed the "butyrate paradox."[2] Collectively, the studies suggest that the cancer preventive benefits of butyrate depend in part on amount, time of exposure (with respect to the tumorigenic process), and the type of fat in the diet. Low carbohydrate diets are known to reduce the amount of butyrate produced in the colon.
Butyric acid has been associated with the ability to inhibit the function of certain (histone deacetylase) enzymes. It is thought that butyric acid enhances the production of RNA from DNA sites (promoters) that are typically silenced/downregulated by the activity of histone deacetylase.
See also
Notes
- ↑ IUPAC is the acronym for the International Union of Pure and Applied Chemists.
- ↑ Joanne Lupton. "Microbial Degradation Products Influence Colon Cancer Risk: the Butyrate Controversy." The Journal of Nutrition, 134, 2004: 479. Retrieved September 12, 2007.
ReferencesISBN links support NWE through referral fees
- McMurry, John. 2004. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-643669-2
- Solomons, T.W. Graham, and Fryhle, Craig B. 2004. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998
- This article incorporates information from the 1911 Encyclopaedia Britannica.
| ||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.