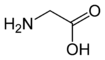
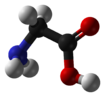
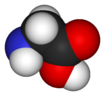
Glycine
| |
Glycine | |
| Systematic (IUPAC) name | |
| aminoethanoic acid | |
| Identifiers | |
| CAS number | 56-40-6 |
| PubChem | 750 |
| Chemical data | |
| Formula | C2H5NO2 |
| Mol. weight | 75.07 |
| SMILES | NCC(O)=O |
| Complete data | |
Glycine is one of the 20 most common, natural, "proteinogenic" (literally, protein building) standard amino acids. It is the simplest of the amino acids in terms of molecular structure and unique among the standard amino acids in that is not optically active; that is, its molecular structure looks the same in its mirror image reflection (unlike the other standard amino acids whose mirror-image reflection forms are distinctively different like right and left hand gloves).
Glycine is necessary for normal functioning in humans and can be synthesized by the human body from other compounds. It is categorized as a non-essential amino acid because it does not have to be taken in with the diet, .
Glycine is important for the synthesis of not only proteins, but also purines (an essential component of DNA and RNA), porphyrins (an essential component of hemoglobin in blood and of the pigment giving meat its red color), ATP, serine, and numerous other organic chemicals, and is a major inhibitory neurotransmitter in the central nervous system.
Most proteins contain only small quantities of glycine. A notable exception is collagen, which contains about one-third glycine. Glycine is also prominent in gelatin. Glycine and alanine, two small amino acids, form the bulk of the protein comprising spider silk, one of the strongest materials known, comparable to high-grade steel, but considerably less dense. The glycine units also are responsible for the elasticity of spider silk. The unique arrangement of the glycine and alanine subunits gives silk its remarkable characteristics and is an example of the wonder and harmonious coordination in nature. Scientists are studying spider silk in the hope of learning how to replicate such an extraordinary fiber.
Glycine's three letter code is GLY, its one letter code is G, and its codons are GGU, GGC, GGA and GGG (IUPAC-IUB 1983). Its systematic name is aminoethanoic acid.
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate (COOH) groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R | H2N-C-COOH | H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. Glycine, however, because of its simple structure and two hydrogen atoms at the α carbon, does not have D- and L-stereoisomers. It is unique among the 20 standard amino acids in not being optically active.
Glycine, the smallest α–amino acid, is structurally simple (having a side chain of just hydrogen), rotates easily, and adds flexibility to the protein chain. It is able to fit into the tightest spaces, e.g., the triple helix of collagen. Because of its structural simplicity, this compact amino acid tends to be evolutionarily conserved in, for example, cytochrome c, myoglobin, and hemoglobin.
Glycine has the chemical formula CH2(NH2)-COOH, or more generally, C2H5NO2.
Biosynthesis
Glycine is not essential to the human diet, since it is synthesized in the body. It is biosynthesized from the amino acid serine. The enzyme serine hydroxymethyl transferase catalyses this transformation (Lehninger et al. 2000):
- HO2CCH(NH2)CH2OH + H2folate → HO2CCH2NH2 + CH2-folate + H2O
Physiological function
In addition to being the building block of proteins, glycine is a building block to numerous other chemical compounds. For one, glycine is one of the reactants in the synthesis of porphyrins such as heme, which is a component of the hemoglobin molecules found in red blood cells. Specifically, aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinoyl coenzyme A. Glycine provides the central C2N subunit of all purines (Lehninger et al. 2000).
Glycine also is important in the biosynthesis of the amino acid serine and the coenzyme glutathione.
Glycine is one of the major inhibitory neurotransmitters in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an inhibitory postsynaptic potential (IPSP). Strychnine is an antagonist at ionotropic glycine receptors. Glycine is a required co-agonist, along with glutamate, for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behavior is facilitated at the (NMDA) glutaminergic receptors which are excitatory. The LD50 of glycine is 7930 mg/kg in rats (oral) (PTCL 2006), and it usually causes death by hyperexcitability.
Glycine is also important as a additive for animal feed, used to reduce saccharin's bitter taste, and used in biochemical research and medicine.
Silk
Glycine is a key component in silk. Spider silk is a remarkably strong material, with a tensile strength comparable to that of high-grade steel (Shao and Vollrath 2002). It has been said that a circular web, similar in all ways to that found in nature but the size of a football field, could stop a commercial jetliner in mid flight (Henke 2007), and yet spider silk is so light that a single strand long enough to circle the earth would weigh less than 16 ounces (460 g).
Spider dragline silk is made up of the protein fibroin, which is a combination of the proteins spidroin one and spidroin two. The bulk of these proteins are made up of glycine (Gly) and alanine (Ala), with the remaining components mostly the amino acids proline (Pro), tyrosine (Tyr), arginine (Arg), glutamine (Gln), serine (Ser), and leucine (Leu) (UB 2007). Spidroin one and two are made up of polyalanine regions with about four to nine alanine monomers in a block (van Beek et al. report approximately eight monomers) and glycine rich areas with a sequence of five amino acids continuously repeated, such as Gly-Pro-Gly-Gln-Gln (van Beek et al. 2002; UB 2007).
The general trend in spider silk structure thus is a sequence of amino acids (usually alternating glycine and alanine, or alanine alone) that self-assemble into a beta sheet conformation. These "Ala rich" blocks are separated by segments of amino acids with bulky side-groups. The beta sheets stack to form crystals, whereas the other segments form amorphous domains. It is the interplay between the hard crystalline segments, and the elastic semi- amorphous regions that gives spider silk its extraordinary properties. The fact that the major amino acids in spider silk are the two smallest amino acids, and lack bulky side groups, allows them to pack together tightly (UB 2007).
The glycine-rich regions give spider silk its elasticity, as each sequence of five amino acids is followed by a 180 degree turn, resulting in a spiral. Capture silk is the most elastic, with about 43 repeats on average, and can extend two to four times its original length, while dragline silk only repeats about nine times and can extend about 30 percent of original length (UB 2007).
ReferencesISBN links support NWE through referral fees
- Dawson, R. M. C., D. C. Elliott, W. H. Elliott, and K. M. Jones. 1986. Data for Biochemical Research, 3rd edition. Oxford: Clarendon Press. ISBN 0198553587.
- Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press. ISBN 0306431319.
- Henke, B. 2007. The webs they weave Poststar.com, June 10, 2007. Retrieved November 25, 2007.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology IUPAC-IUB. Retrieved November 25, 2007.
- Kendall, E. C., and B. F. McKenzie. 1941. dl-Alanine Organic Syntheses 1: 21. Retrieved November 25, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
- Physical & Theoretical Chemistry Laboratory (PTCL). 2006. Safety (MSDS) data for glycine The Physical and Theoretical Chemistry Laboratory, Oxford University.
- Shao, Z., and F. Vollrath. 2002. Surprising strength of silkworm silk. Nature 418: 741.
- University of Bristol, School of Chemistry (UB). 2007. Spider silk: Chemical structure University of Bristol. Retrieved November 25, 2007.
- van Beek, J. D., S. Hess, F. Vollrath, and B. H. Meier. 2002. The molecular structure of spider dragline silk: Folding and orientation of the protein backbone Proc Natl Acad Sci USA 99(16): 10266-10271. Retrieved November 25, 2007.
External links
All links retrieved May 23, 2024.
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.