Peptide
Peptides are short chains of amino acids linked together via peptide bonds and having a defined sequence. Peptides function primarily as signaling molecules in animals or as antibiotics in some lower organisms.
The number of amino acid molecules present in a peptide is indicated by a prefix. For example, a dipeptide has two amino acids; a tripeptide has three. An oligopeptide contains a few molecules; a polypeptide contains many. Peptides generally contain fewer than 30 amino acid residues, while polypeptides contain as many as 4000. The distinction between polypeptides and proteins is largely academic and imprecise, and the two terms are sometimes used interchangeably. However, there is a movement within the scientific community to define proteins as polypeptides (or complexes of polypeptides) with three-dimensional structure.
In animals, peptides are involved in the complex coordination of the body, with three major classes of peptides involved in signaling:
- Peptide hormones, which function as chemical messengers between cells. Growth hormone, for example, is involved in the general stimulation of growth, and insulin and glucagon are well known peptide hormones.
- Neuropeptides, which are peptides found in neural tissue. Endorphins and enkephalins are neuropeptides that mimic the effects of morphine, inhibiting the transmission of pain signals. The peptides vasopressin and oxytoxin have been linked to social behaviors such as pair-bonding.
- Growth factors, which play a role in regulating animal cell growth and differentiation.
Human creativity has led to peptides being important tools for understanding protein structure and function. Peptide fragments are components of proteins that researchers use to identify or quantify the source protein. Often these fragments are the products of enzymatic degradation performed in the laboratory on a controlled sample, but they can also be forensic or paleontological samples that have been degraded by natural effects. Peptides also allow antibodies to be generated without the need to purify the protein of interest, by making antigenic peptides of sections of the protein.
The components of peptides
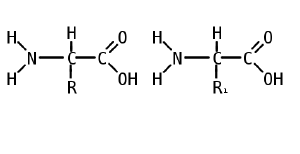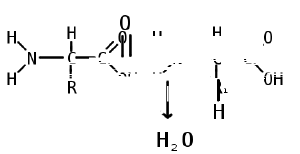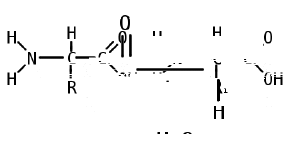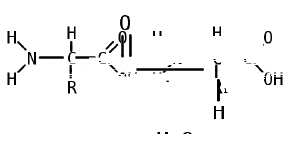
Like proteins, peptides are built from combinations of 20 different amino acids, which are organic molecules composed of an amino group (-NH2), a carboxylic acid group (-COOH), and a unique R group, or side chain. Two amino acids (specifically, alpha-amino acids) are linked together by a peptide bond. A peptide bond is a chemical bond formed between two molecules when the carboxyl group of one amino acid reacts with the amino group of the other amino acid; the resulting CO-NH bond is called a peptide bond. An amino acid residue is what is left of an amino acid once it has coupled with another amino acid to form a peptide bond.
Peptides are then created by the polymerization of amino acids, a process in which amino acids are joined together in chains. Shorter strings of amino acids may be referred to as peptides, or, less commonly, oligopeptides.
Peptide synthesis
Peptides are synthesized from amino acids according to an mRNA template, which is itself synthesized from a DNA template inside the cell's nucleus. The precursors of ribosomal peptides are processed in several stages in the endoplasmic reticulum, resulting in "propeptides." These propeptides are then packaged into membrane-bound secretory vesicles, which can be released into the bloodstream in response to specific stimuli.
Nonribosomal peptides, found primarily in fungi, plants, and, unicellular organisms are synthesized using a modular enzyme complex (which functions much like a conveyor belt in a factory). All of these complexes are laid out in a similar fashion, and they may contain many different modules to perform a diverse set of chemical manipulations on the developing peptide. Nonribosomal peptides often have highly complex cyclic structures, although linear nonribosomal peptides are also common.
Some key peptide groups and their biological function
Peptides comprise the widest variety of signaling molecules in animals. The three major classes of peptides are peptide hormones, neuropeptides, and polypeptide growth factors. Many peptides are found in both the brain and non neural tissues. The blood-brain barrier prevents peptide hormones traveling in the blood from entering the brain, so that they do not interfere with the functioning of the central nervous system.
Peptide hormones
Peptide hormones are a class of peptides that function in living animals as chemical messengers from one cell (or group of cells) to another. Well-known peptide hormones include insulin, glucagon, and the hormones secreted from the pituitary gland, an endocrine gland about the size of a pea that sits in a small, bony cavity at the base of the brain. The latter include follicle stimulating hormone (FSH), growth hormone, and vasopressin. However, peptide hormones are produced by many different organs and tissues, including the heart, pancreas, and gastrointestinal tract.
Neuropeptides
A neuropeptide is any of the variety of peptides found in neural tissue. Approximately 100 different peptides are currently known to be released by different populations of neurons in the mammalian brain. Some neuropeptides act both as neurotransmitters in the nervous system and as neurohormones that act on distant cells.
Neurons use many different chemical signals to communicate information, including neurotransmitters, peptides, cannabinoids, and even some gases, like nitric oxide. Peptide signals play a role in information processing distinct from that of conventional neurotransmitters. While neurotransmitters generally affect the excitability of other neurons by depolarizing them or hyperpolarizing them, peptides have much more diverse effects; among other things, they can affect gene expression, local blood flow, and the formation of synapses.
Neurons very often produce both a conventional neurotransmitter (such as glutamate, GABA or dopamine) and one or more neuropeptides. Peptides are generally packaged in large dense-core vesicles, while the co-existing neurotransmitters are contained in small synaptic vesicles.
Vasopressin and oxytoxin
The neuropeptide Arginine vasopressin (AVP), also known as argipressin or antidiuretic hormone (ADH), is a hormone found in humans. It is mainly released when the body is low on water; it stimulates water reabsorption in the kidneys. It performs diverse actions when released in the brain, and has been implicated in memory formation, aggression, blood pressure regulation, and temperature regulation. Similar vassopressins are found in other mammalian species.
In recent years, there has been particular interest in the role of vasopressin in social behavior. It is thought that vasopressin, released into the brain during sexual activity, initiates and sustains patterns of activity that support the pair-bond between the sexual partners; in particular, vasopressin seems to induce the male to become aggressive towards other males. Evidence for this connection comes from experimental studies on several species which indicate that the precise distribution of vasopressin and vasopressin receptors in the brain is associated with species-typical patterns of social behavior. In particular, there are consistent differences between monogamous species and promiscuous species in the distribution of vasopressin receptors, and sometimes in the distribution of vasopressin-containing axons, even when closely-related species are compared. Moreover, studies involving either injecting vasopressin agonists into the brain or blocking the actions of vasopressin support the hypothesis that vasopressin is involved in aggression towards other males. There is also evidence that differences in the vasopressin receptor gene between individual members of a species might be predictive of differences in social behavior.
Oxytocin is a mammalian hormone involved in the stimulation of smooth muscle contraction that also acts as a neurotransmitter in the brain. In women, it is released mainly after distension of the cervix and vagina during labor, and after stimulation of the nipples, facilitating birth and breastfeeding, respectively.
Opioid peptides
Opioid peptides produced in the body include endorphins and enkephalins. Opioid peptides act as natural pain killers, or opiates, decreasing pain responses in the central nervous system.
Growth factors
Polypeptide growth factors control animal cell growth and differentiation. Nerve growth factor (or NGF) is involved in the development and survival of neurons, while platelet-derived growth factor (PDGF) participates in blood clotting at the site of a wound. PDGF stimulates the spread of fibroblasts in the vicinity of the clot, facilitating the regrowth of the damaged tissue.
Given the role of polypeptide growth factors in controlling cell proliferation, abnormalities in growth factor signaling are the basis for a variety of diseases, including many types of cancer.
Peptides are an important research tool
Peptides have received prominence in molecular biology in recent times for several reasons:
- Peptides allow researchers to generate antibodies in animals without the need to purify the protein of interest. The researcher can simply make antigenic peptides of sections of the protein.
- Peptides have become instrumental in mass spectrometry, allowing the identification of proteins of interest based on peptide masses and sequences.
- Peptides have recently been used in the study of protein structure and function. For example, synthetic peptides can be used as probes to determine where protein-peptide interactions occur.
- Inhibitory peptides are also used in clinical research to examine the effects of peptides on the inhibition of cancer proteins and other diseases.
Peptide families
Below is a more detailed list of the major families of ribosomal peptides:
- Vasopressin and oxytocin
- Vasopressin
- Oxytocin
- The Tachykinin peptides
- Substance P
- Kassinin
- Neurokinin A
- Eledoisin
- Neurokinin B
- Vasoactive intestinal peptides
- VIP (Vasoactive intestinal peptide)
- PACAP (Pituitary adenylate cyclase activating peptide)
- PHI 27
- PHM 27
- GHRH 1-24 (Growth hormone releasing hormone 1-24)
- Glucagon
- Secretin
- Pancreatic polypeptide-related peptides
- NPY
- PYY (Peptide YY)
- APP (Avian pancreatic polypeptide)
- HPP (Human pancreatic polypeptide)
- Opioid peptides
- Proopiomelanocortin (POMC) Peptides
- The Enkephalin pentapeptides
- The Prodynorphin peptides
- Calcitonin peptides
- Calcitonin
- Amylin
- AGG01
ReferencesISBN links support NWE through referral fees
- Cooper, G. M., and R. E. Hausman. 2004. The Cell: A Molecular Approach, 3rd edition. Washington, DC: ASM Press & Sunderland, MA: Sinauer Associates. ISBN 0878932143
- Lodish, H., D. Baltimore, A. Berk, S. L. Zipursky, P. Matsudaira, and J. Darnell. 1996. Molecular Cell Biology. Oxford: W H Freeman and Company. ISBN 0716727110
- Stryer, L. 1995. Biochemistry, 4th edition. New York, NY: W.H. Freeman. ISBN 0716720094
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Peptide history
- Peptide_bond history
- Peptide_hormone history
- Neuropeptide history
- Vasopressin history
- Opioid_peptide history
- Pituitary_gland history
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.