| Al2(SO4)3 |
|---|
chemical formula for aluminum sulfate |
A chemical formula is a concise way of expressing information about the atoms that constitute a particular chemical compound. It is also useful for showing how a chemical reaction takes place.
For a molecular compound, the chemical formula gives the chemical symbols for the constituent elements and indicates the number of atoms of each element found in each molecule of that compound. If a molecule contains more than one atom of a particular element, this quantity is indicated using a subscript after the chemical symbol (although nineteenth century books often used superscripts). For an ionic compound or other non-molecular substance, the subscripts indicate the ratio of elements in the compound.
Empirical formula
In chemistry, the empirical formula of a chemical is a simple expression of the relative number of each type of atom in the molecule or the ratio of elements in the compound. Empirical formulas are the standard for ionic compounds, such as CaCl2, and for macromolecules, such as SiO2. An empirical formula makes no reference to isomerism, structure, or absolute number of atoms. The term empirical refers to the process of elemental analysis, a technique of analytical chemistry used to determine the relative percent composition of a pure chemical substance by element.
Consider the formula for hydrogen peroxide. Each molecule of hydrogen peroxide consists of two atoms of hydrogen and two atoms of oxygen. Its molecular formula (explained below) would be written as H2O2, but its empirical formula is simply HO, expressing the 1:1 ratio of component elements.
In the case of hexane, each molecule has six atoms of carbon and 14 atoms of hydrogen. The ratio of carbon atoms to hydrogen atoms is 3:7, and its empirical formula is therefore written as C3H7.
Molecular formula
Consider methane, a simple molecule consisting of one carbon atom bonded to four hydrogen atoms. It has the chemical formula:
In this case, the molecular formula is the same as the empirical formula.
A molecule of glucose has six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. Its molecular formula is:
A chemical formula may also supply information about the types and spatial arrangement of bonds in the chemical, though it does not necessarily specify the exact isomer. For example ethane consists of two carbon atoms single-bonded to each other, with each carbon atom having three hydrogen atoms bonded to it. Its chemical formula can be rendered as CH3CH3. If there were a double bond between the carbon atoms (and thus each carbon only had two hydrogens), the chemical formula may be written: CH2CH2, and the fact that there is a double bond between the carbons is assumed. However, a more explicit and correct method is to write H2C:CH2 or H2C=CH2. The two dots or lines indicate that a double bond connects the atoms on either side of them.
A triple bond may be expressed with three dots or lines, and if there may be ambiguity, a single dot or line may be used to indicate a single bond.
Molecules with multiple functional groups that are the same may be expressed in the following way: (CH3)3CH. However, this implies a different structure from other molecules that can be formed using the same atoms (isomers). The formula (CH3)3CH implies a chain of three carbon atoms, with the middle carbon atom bonded to another carbon:
and the remaining bonds on the carbons all leading to hydrogen atoms. However, the same number of atoms (10 hydrogens and 4 carbons, or C4H10) may be used to make a straight chain: CH3CH2CH2CH3.
The alkene but-2-ene has two isomers which the chemical formula CH3CH=CHCH3 does not identify. The relative position of the two methyl groups must be indicated by additional notation denoting whether the methyl groups are on the same side of the double bond (cis or Z) or on the opposite sides from each other (trans or E).
Polymers
For polymers, parentheses are placed around the repeating unit. For example, a hydrocarbon molecule that is described as: CH3(CH2)50CH3, is a molecule with 50 repeating units. If the number of repeating units is unknown or variable, the letter n may be used to indicate this: CH3(CH2)nCH3.
Representation of ions
For ions, the charge on a particular atom may be denoted with a right-hand superscript. For example Na+, or Cu2+. The total charge on a charged molecule or a polyatomic ion may also be shown in this way. For example: hydronium, H3O+ or sulfate, SO42-.
Representation of isotopes
Although isotopes are more relevant to nuclear chemistry or stable isotope chemistry than to conventional chemistry, different isotopes may be indicated with a left-hand superscript in a chemical formula. For example, the phosphate ion containing radioactive phosphorus-32 is 32PO43-. Also, a study involving stable isotope ratios might include 18O:16O.
A left-hand subscript is sometimes used to indicate redundantly, for convenience, the atomic number.
Structural formula
The structural formula of a chemical compound is a graphical representation of the molecular structure showing how the atoms are arranged. The chemical bonding within the molecule is also shown, either explicitly or implicitly. There are three common representations used in publications, condensed, Lewis type, and line-angle formulas. There are also several formats used for structural representation in chemical databases, such as SMILES, InChI, and CML.
Unlike molecular formulas or chemical names, structural formulas provide a very powerful representation of the molecular structure. Chemists nearly always describe a chemical reaction or synthesis using structural formulas rather than chemical names, because the structural formulas allow the chemist to visualize the changes that occur.
Many chemical compounds can exist in different isomeric forms that have different structures but the same overall chemical formula. A structural formula indicates the arrangements of atoms in a way that a chemical formula cannot. A simple example of this may be seen with the hydrocarbon butane, which has the molecular formula C4H10. The four carbons may be arranged in a linear pattern, or in a branched, "T" pattern. The first arrangement is known as orthobutane or n-butane, while the second is isobutane.
Common types of structural formula
There are three main types of structural formula in widespread use in the chemical literature.[1]
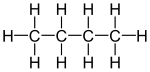
Condensed formula In early organic chemistry publications, where use of graphics was severely limited, a text-based system arose to describe organic structures in a line of text. Although this system tends to break down with complex cyclic compounds such as strychnine, it remains a convenient way to represent simple structures such as ethanol (CH3CH2OH).
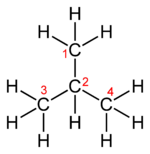
Skeletal formula Note that for organic compounds, line drawings of structural formula are assumed to have carbon atoms at the vertices and termini of all line segments not marked with the atomic symbol of an element (other than carbon). Each carbon atom is in turn assumed to bear enough hydrogen atoms to give the carbon atom four bonds. Equivalent full and abbreviated forms are shown in the adjacent figures.
A chemical structure can be precisely, uniquely, and unambiguously described using IUPAC nomenclature. In the case of isobutane, the IUPAC systematic name is methylpropane.
Multiple planes When substituents of a molecule exist in different planes, their position can be described using solid and dotted wedges, with the former showing a substituent coming out of the plane, and the latter going into it. This system is useful in describing differences between chiral molecules.
Non-stoichiometric formulas
Chemical formulas most often use natural numbers for each of the elements. However, there is a whole class of compounds, called non-stoichiometric compounds, that cannot be represented by well-defined natural numbers. Such a formula might be written using real numbers, as in Fe0.95O, or it might include a variable part represented by a letter, as in Fe1âxO.
General forms for organic compounds
The chemical formula used for a series of compounds that differ from each other by a constant unit is called a general formula. Such a series is called a homologous series, and its members are called homologs.
Hill system
The Hill system is a system of writing chemical formulas such that the number of carbon atoms in a molecule is indicated first, the number of hydrogen atoms next, and then the number of all other chemical elements subsequently, in alphabetical order. When the formula contains no carbon, all the elements, including hydrogen, are listed alphabetically.
By sorting formulas according to the number of atoms of each element present in the formula according to these rules, with differences in earlier elements or numbers being treated as more significant than differences in any later element or numberâlike sorting text strings into lexicographic orderâit is possible to collate chemical formulas into what is known as Hill system order.
The Hill system was first published by Edwin A. Hill of the United States Patent Office in 1900.
Examples
The following formulas are written using the Hill system, and listed in Hill order:
- BrH
- BrI
- CH3I
- C2H5Br
- HI
Notes
- â L. G. Wade, Organic Chemistry, 4th ed (Upper Saddle River, NJ: Prentice Hall, 1999, ISBN 978-0139227417), 17-20.
ReferencesISBN links support NWE through referral fees
- Brown, Theodore E., H. Eugene LeMay, and Bruce E. Bursten. Chemistry: The Central Science, 10th edition. Upper Saddle River, NJ: Prentice Hall, 2005. ISBN 0131096869
- Chang, Raymond. Chemistry, 9th edition. New York: McGraw-Hill Science/Engineering/Math, 2006. ISBN 0073221031
- Housecroft, Catherine E. and Alan G. Sharpe. Inorganic Chemistry, 4th edition. Harlow, UK: Prentice Hall, 2001. ISBN 0582310806
- McMurry, John and Robert C. Fay. 2004. Chemistry, 4th edition. Upper Saddle River, NJ: Prentice Hall. ISBN 0131402080
- Moore, John W., Conrad L. Stanitski, and Peter C. Jurs. Chemistry: The Molecular Science. New York: Harcourt College, 2002. ISBN 0030320119
- Smith, Roland. Conquering Chemistry. Sydney: McGraw-Hill, 1994. ISBN 0074701460
- Wade, L. G. Organic Chemistry. Upper Saddle River, NJ: Prentice Hall, 1999. ISBN 978-0139227417
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.