Fruit fly may refer to:
- Tephritidae, the family of large fruit flies.
- Drosophilidae, the family of small fruit flies or vinegar flies, including:
- Drosophila melanogaster, often called the common fruit fly, and an important model organism in modern biology.
These are all members of the Diptera order of the Insecta class of the phylum Arthropoda. Insects of the order Diptera, known as true flies, are characterized by having a single pair of true wings, which are used for flight, while the hind wings are reduced into a pair of small knob-like structures called the halteres.
Fruit flies are of major importance. On the positive side, they can serve as agents for biological control of pests, and the species Drosophilia melanogaster, as a major model organism for research, is unlocking secrets related to genetics and even certain diseases in humans. On the negative side, fruit flies can be a major agricultural pest, with the potential to destroy up to 100 percent of some crops.
For the latter reason, countries without fruit flies infestations may impose strong quarantine restrictions or even bans on fruit imported from countries where the fruit fly is endemic. In caring for nature, the human responsibility extends to exhibiting utmost concern regarding introduction of foreign species to new areas. History is replete with cases of invasive species (Mediterranean fruit fly, sea lamprey, ctenophore Mniopsis leidyi, gypsy moth, etc.) wreaking havoc in the ecosystems into which they have been introduced, either purposefully or accidentally.
Tephritidae
| Tephritidae | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Male Paracantha cultaris | ||||||||||||||||
| Scientific classification | ||||||||||||||||
| ||||||||||||||||
| Genera | ||||||||||||||||
|
500 genera & |
Tephritidae is a family of insects that includes large fruit flies. It does not include the biological model organism of the genus Drosophila, which is often called the common fruit fly. There are nearly five thousand described species of tephritid fruit fly, categorized in almost five hundred genera. Description, recategorization, and genetic analysis have often changed the taxonomy of the fruit fly family.
Tephritid fruit flies are of major importance in agriculture. Some have negative effects, some positive. Various species of fruit fly cause damage to fruit and other plant crops. The genus Bactrocera is of worldwide notoriety for its destructive impact on agriculture. The olive fruit fly (B. oleae), for example, feeds on only one plant: the wild or commercially cultivated olive. It has the capacity to ruin one hundred percent of an olive crop by damaging the fruit. On the other hand, some fruit flies are used as agents of biological control, as they reduce the populations of pest species. Several species of the fruit fly genus Urophora have been shown to be effective agents against rangeland-destroying noxious weeds, such as starthistles and knapweeds.
Most fruit flies lay their eggs in plant tissues, where the larvae find their first food upon emerging. The adults usually have a very short lifespan, some living for less than a week.
Fruit flies have an open circulatory system as its cardiovascular system.
Their behavioral ecology is of great interest to biologists. Some fruit flies have extensive mating rituals or territorial displays. Many are brightly colored and visually showy. Some fruit flies show Batesian mimicry, bearing the colors and markings of dangerous insects, such as wasps, because it helps the fruit flies to avoid predators; the flies, of course, lack stingers.
Economically important Tephritidae include:
- Mediterranean fruit fly Ceratitis capitata (Wiedemann)
- Olive fruit fly Bactrocera oleae (Gmelin)
- Queensland fruit fly Bactrocera tryoni
Drosophilidae
| Drosophilidae | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drosophilia melanogaster | ||||||||||||||||
| Scientific classification | ||||||||||||||||
|
Drosophilidae is a diverse family of flies, including the genus Drosophila, which includes fruit flies, vinegar flies, wine flies, pomace flies, grape flies, and picked fruit-flies. The best known species is Drosophila melanogaster, which is used extensively for studies concerning genetics, development, physiology, ecology and behavior.
Drosophila is a genus of small flies whose members are often called small fruit flies, or more appropriately vinegar flies, wine flies, pomace flies, grape flies, and picked fruit-flies. The terms "fruit fly" and "Drosophila" are often used synonymously with Drosophila melanogaster in modern biological literature. The entire genus, however, contains about 1,500 species and is very diverse in appearance, behavior, and breeding habitat.
A recent version of the diagnostic characteristics can be found in Drosophila: A Laboratory Handbook by Ashburner et al. (2005).
The knowledge of the phylogeny of this family is incomplete. The family is subdivided in two subfamilies, the Drosophilinae and the Steganinae. The two subfamilies do not contain a single morphological character that distinguishes them. However, the combination of characteristics is sufficient to assign species correctly to the subfamilies. Most molecular phylogeny studies focus on the genus Drosophila and related genera.
Drosophilia melanogaster
| Drosophila melanogaster | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
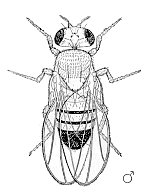 Male Drosophila melanogaster | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Drosophila melanogaster Johann Wilhelm Meigen, 1830 |
Drosophila melanogaster (from Greek, meaning black-bellied dew-lover) is a two-winged insect that belongs to the Diptera, the order of true flies. The species is commonly known as the fruit fly, and is one of the most commonly used model organisms in biology, including studies in genetics, physiology and life-history evolution. Flies belonging to the Tephritidae are also called fruit flies, which often leads to confusion.
Physical appearance
Drosophila have red eyes and black rings across their abdomen. They exhibit sexual dimorphism: females are about 2.5 millimeters long; males are slightly smaller and the back of their bodies is darker.
Males are easily distinguished from females based on color differences: males have a distinct black patch at the bottom of the abdomen, less noticeable in recently emerged flies (see fig). Male flies have sexcombs, or a row of dark bristles on the tarsus of the first leg. (In flies, tarsus generally refers to the distal section of a limb). Furthermore, males have a cluster of spiky hairs (claspers) surrounding the anus and genitals used to attach to the female during mating.
Drosophila flight
The wings of a fly are capable of beating at up to 220 times per second. Flies fly via straight sequences of movement interspersed by rapid turns called saccades. During these turns, a fly is able to rotate 90 degrees in fewer than 50 milliseconds.
Drosophila, and probably many other flies, have optic nerves which lead directly to the wing muscles (while in other insects they always lead to the brain first), making it possible for them to react even more quickly than most insects.
It was long thought that the characteristics of Drosophila flight were dominated by the viscosity of the air, rather than the inertia of the fly body. However, recent research by Michael Dickinson and Rosalyn Sayaman has indicated that flies perform banked turns, where the fly accelerates, slows down while turning, and accelerates again at the end of the turn. This indicates that inertia is the dominant force, as is the case with larger flying animals.
Drosophila genome
The genome of Drosophila contains four pairs of chromosomes: an X/Y pair and three autosomes labeled 2, 3, and 4. The fourth chromosome is so tiny that it is often ignored, aside from its important eyeless gene. The genome contains about 132 million bases and approximately 13,767 genes. The genome has been sequenced and has been annotated. Determination of sex in Drosophila occurs by the ratio of X chromosomes to autosomes, not because of the presence of a Y chromosome as in human sex determination.
Genetically, humans are about 44 percent similar to flies. About 61 percent of known human disease genes have a recognizable match in the genetic code of fruit flies, and 50 percent of fly protein sequences have mammalian analogs. Drosophila is being used as a genetic model for several human diseases including the neurodegenerative disorders Parkinson's disease, Huntington's disease, and Alzheimer's disease. The fly is also being used to study mechanisms underlying immunity, diabetes, cancer, and drug abuse.
Life cycle
The developmental period for Drosophila melanogaster varies with temperature, as it does with all cold-blooded species. The shortest development time (egg to adult) is seven days at 28 °C (Ashburner and Thompson 1978, Ashburner et al. 2005). Development times increase at higher temperatures (30 °C, 11 days) due to heat stress. Under ideal conditions, the development time at 25 °C is 8.5 days (Bloomington 2006, Ashburner and Thompson 1978, Ashburner et al. 2005), at 18 °C it takes 19 days (Ashburner and Thompson 1978, Ashburner et al. 2005), and at 12 °C it takes over 50 days (Ashburner and Thompson 1978, Ashburner et al. 2005). Under crowded conditions, development time increases (Chiang 1950), while the emerging flies are smaller (Chiang 1950, Bakker 1961).
Females lay some four hundred eggs (embryos), about five at a time, into rotting fruit or other suitable material, such as decaying mushrooms and sap fluxes. The eggs, which are about 0.5 millimeters long, hatch after 12-15 hours (at 25 °C) (Ashburner and Thompson 1978; Ashburner et al. 2005). The resulting larvae grow for about 4 days (at 25 °C) while molting twice (into 2nd- and 3rd-instar larvae), at about 24 and 48 hours after eclosion (Ashburner and Thompson 1978; Ashburner et al. 2005). During this time, they feed on the microorganisms that decompose the fruit, as well as on the sugar of the fruit themselves. Then the larvae encapsulate in the puparium and undergo a four-day-long metamorphosis (at 25 °C), after which the adults eclose (emerge) (Ashburner and Thompson 1978; Ashburner et al. 2005).
Females become receptive to courting males at about 8-12 hours after emergence (Pitnick 1996). Males perform a sequence of five behavioral patterns to court females. First males orient themselves while playing a courtship song by horizontally extending and vibrating their wings. Soon after the male positions itself at the rear of the female's adbdomen in a low posture to tap and lick the female genitalia. Finally the male curls its abdomen, and attempts copulation. Females can reject males by moving away from males and extruding their ovipositor (the ovipositor being the last segments of the female fly's thorax, formed into a pointy shape). The average duration of copulation, when successful, lasts ten minutes, during which males transfer hundreds of very long sperm in seminal fluid to the female. Females store the sperm, which may need to compete with other males' stored sperm to fertilize eggs.
Development and embryogenesis
Embryogenesis in Drosophila has been extensively studied, as its small size, short generation time, and large brood size makes the fly ideal for genetic studies. Drosophila are also unique among model organisms in that cleavage occurs in a syncytium, which is a cell with many nuclei resulting from nuclear division unaccompanied by cytokinesis (division of the cytoplasm).
After fertilization of the oocyte, the early embryo or (syncytial embryo) undergoes rapid DNA replication and 13 nuclear divisions until approximately five thousand to six thousand nuclei accumulate in the unseparated cytoplasm of the embryo. By the end of the eighth division, most nuclei have migrated to the surface, surrounding the yolk sac (or the first gestational sac that appears during pregnancy), leaving behind only a few nuclei, which will become the yolk nuclei. After the tenth division the pole cells form at the posterior end of the embryo, separating the germ line from the syncytium. Finally, after the thirteenth division cell membranes slowly invaginate (fold inward), dividing the syncytium into individual somatic cells. The end of this process marks the beginning of gastrulation, the state in development when the three germ layers develop and the mature form of the individual begins to take shape.
Nuclear division in the early Drosophila embryo happens so quickly that there are no proper checkpoints, and as a result DNA is easily damaged. When the DNA is damaged, the nuclei containing damaged DNA detach from their centrosomes (which are responsible for mitotic spindle formation) and fall into the center of the yolk sac. Because this section does come to not form part of the fly, the DNA damage does not cause problems for the developing individual.
The gene network (transcriptional and protein interactions) governing the early development of the fruit fly embryo is one of the most understood gene networks to date, especially relating to patterning along the antero-posterior (AP) and dorso-ventral (DV) axes.
The egg undergoes well-characterized morphogenetic movements during gastrulation and early development, including germ-band extension, formation of several furrows, ventral invagination of the mesoderm, posterior and anterior invagination of endoderm (gut), as well as extensive body segmentation.[1] Finally the egg hatches from the surrounding cuticle into a first instar larva. During the larval development (referred to as molting), the imaginal disks form, which are in essence the anlagen (precursor) for the entire adult body. Cells of the imaginal disks are set aside early on and they mature over time into adult body structures, especially during pupation. In contrast, most other cells in the larva undergo apoptosis.
Model organism in genetics
Drosophila melanogaster is one of the most studied organism in biological research, particularly in genetics and developmental biology. There are several reasons:
- It is small and easy to grow in the laboratory
- It has a short generation time (about two weeks) and high productivity (females can lay five hundred eggs in ten days)
- The mature larvae show giant chromosomes in the salivary glands called polytene chromosomes, and the formation of "puffs" here indicate regions of transcription and hence gene activity.
- It has only four pairs of chromosomes: three autosomal, and one sex.
- Males do not show recombination, facilitating genetic studies.
- Genetic transformation techniques have been available since 1987.
- Its compact genome was sequenced in 1998 (Adams 2000).
Charles W. Woodworth is credited with being the first to breed Drosophila in quantity and for suggesting to W. E. Castle that they might be used for genetic research during his time at Harvard University. Beginning in 1910, fruit flies helped Thomas Hunt Morgan accomplish his studies on heredity. Morgan described X-linked inheritance, which confirmed that genes exist on chromosomes. He also showed that genes located on the same chromosome do not show independent assortment. His studies of linked traits led to the first maps showing the locations of genetic loci on chromosomes. The first maps of Drosophila chromosomes were completed by Alfred Sturtevant.
Neuroscience and behavioral genetics
In 1971 Ron Konopka and Seymour Benzer published a paper titled "Clock mutants of Drosophila melanogaster" in which they described mutations that affected an animal's behavior. Wild-type flies show a regular 24-hour activity rhythm (i.e. circadian rhythm, as the rhythm has a period of about one day, stays constant under constant conditions, and varies little with temperature). Konopka and Benzer found mutant flies that had increased and decreased activity rhythms, as well as some with broken rhythms (flies that move and rest in random spurts). Work over the following thirty years has shown that these mutations and others like them affect a group of genes and their products that comprise a biochemical or biological clock. This clock is found in a wide range of fly cells, but the clock-bearing cells that control activity are several dozen neurons in the fly's central brain.
Since then Benzer, his students, and many others have used behavioral screens to isolate genes involved in vision, olfaction, audition, learning and memory, courtship, pain and other processes such as longevity. Drosophila has also been used in neuropharmacological research.
Vision in Drosophila
The compound eye of the fruit fly contains eight hundred unit eyes or ommatidia, and is one of the most advanced eye structures among insects. Each ommatidium contains eight photoreceptor cells (R1-8), support cells, pigment cells, and a cornea. Wild type flies have reddish pigment cells, which serve to absorb excess blue light so that the fly is not blinded by ambient light.
Each photoreceptor cell consists of two main sections, the cell body and the rhabdomere. The cell body contains the nucleus while the rhabdomere is made up of toothbrush-like stacks of membrane called microvilli. Each microvillus is 1-1.5 millimeters in length and fifty nm in diameter. The membrane of the rhabdomere is packed with about 100 million rhodopsin molecules, the visual protein that absorbs light. The rest of the visual proteins are also tightly packed into the microvillar space, leaving little room for cytoplasm.
The photoreceptors in Drosophila express a variety of rhodopsin isoforms. The R1-R6 photoreceptor cells express Rhodopsin1 (Rh1) which absorbs blue light (480 nanometers). The R7 and R8 cells express a combination of either Rh3 or Rh4, which absorb UV light (345-375 nanometers), and Rh5 or Rh6, which absorb blue (437 nanometers) and green (508 nanometers) light respectively. Each rhodopsin molecule consists of an opsin protein covalently linked to a carotenoid chromophore, 11-cis-3-hydroxyretinal.
As in vertebrate vision, visual transduction in invertebrates occurs via a G protein-coupled pathway. However, in vertebrates, the G protein is transducin, while the G protein in invertebrates is Gq (dgq in Drosophila). When rhodopsin (Rh) absorbs a photon of light, its chromophore, 11-cis-3-hydroxyretinal, is isomerized to all-trans-3-hydroxyretinal. Rh undergoes a conformational change into its active form, metarhodopsin. Metarhodopsin activates Gq, which in turn activates a phospholipase Cβ (PLCβ) known as NorpA.
PLCβ hydrolyzes phosphatidylinositol (4,5)-bisphosphate ((PIP2)), a phospholipid found in the cell membrane, into soluble inositol triphosphate (IP3) and diacylgycerol (DAG), which stays in the cell membrane. DAG, or a derivative of DAG, causes a calcium selective ion channel known as transient receptor potential (TRP) to open and calcium and sodium flows into the cell. IP3 is thought to bind to inositol triphosphate receptor (IP3 receptors) in the subrhabdomeric cisternae, an extension of the endoplasmic reticulum, and cause release of calcium, but this process does not seem to be essential for normal vision.
Calcium binds to proteins such as calmodulin (CaM) and an eye-specific protein kinase C (PKC) known as InaC. These proteins interact with other proteins and have been shown to be necessary for turning off the light response. In addition, proteins called arrestins bind metarhodopsin and prevent it from activating more Gq.
A potassium-dependent sodium/calcium exchanger known as NCKX30C pumps the calcium out of the cell. It uses the inward sodium gradient and the outward potassium gradient to extrude calcium at a stoichiometry of 4 Na+/ 1 Ca++, 1 K+.
TRP, InaC, and PLC form a signaling complex by binding a scaffolding protein called InaD. InaD contains five binding domains called PDZ domains which specifically bind the C termini of target proteins. Disruption of the complex by mutations in either the PDZ domains or the target proteins reduces the efficiency of signaling. For example, disruption of the interaction between InaC, the protein kinase C, and InaD results in a delay in inactivation of the light response.
Unlike vertebrate metarhodopsin, invertebrate metarhodopsin can be converted back into rhodopsin by absorbing a photon of orange light (580 nanometers).
Approximately two-thirds of the Drosophila brain (about 200,000 neurons total) is dedicated to visual processing. Although the spatial resolution of their vision is significantly worse than that of humans, their temporal resolution is approximately ten times better.
Notes
- ↑ FlyMove - Stages Frameset. Retrieved May 15, 2007.
ReferencesISBN links support NWE through referral fees
- Adams, M. D., et. al. Science 287 (2000): 2185-2195.The genome sequence of Drosophila melanogaster. Retrieved October 18, 2022.
- Ashburner, M., and J. N. Thompson. The laboratory culture of Drosophila. In M. Ashburner and T. R. F. Wright (eds.). The genetics and biology of Drosophila Volume 2A (1978): 1-81. Academic Press.
- Ashburner, M., K. G. Golic, and R. S. Hawley. Drosophila: A Laboratory Handbook. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2005.
- Bakker, K. "An analysis of factors which determine success in competition for food among larvae of Drosophila melanogaster." Archives Neerlandaises de Zoologie 14 (1961): 200-281.
- Bloomington Drosophilia Stock Center at Indiana University (Bloomington), 2006.
- Chiang, H. C., and A. C. Hodson. "An analytical study of population growth in Drosophila melanogaster." Ecological Monographs 20 (1950): 173-206.
- Fry, S., and M. Dickinson. "The aerodynamics of free-flight manuevers in Drosophila." Science 300 (5618) (2003): 495-498. PMID 12702878
- Haug-Collet, K., et. al. "Cloning and characterization of a potassium-dependent sodium/calcium exchanger in Drosophila." J. Cell Biol. 147 (3) (1999): 659-670. PMID 10545508
- Pitnick, S. "Investment in testes and the cost of making long sperm in Drosophila." American Naturalist 148 (1996): 57-80.
- Raghu, P., et. al. "Normal phototransduction in Drosophila photoreceptors lacking an InsP3 receptor gene." Molec. & Cell. Neurosci. 15 (5) (2000): 429-445. PMID 10833300
- Ranganathan, R., et. al. "Signal transduction in Drosophila photoreceptors." Annu. Rev. Neurosci. 18 (1995): 283-317. PMID 7605064
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.