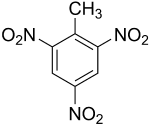
Trinitrotoluene
| Trinitrotoluene | |
|---|---|
| |
| General | |
| Name | Trinitrotoluene |
| Other Names | 2-Methyl-1,3,5-trinitrobenzene 2,4,6-Trinitrotoluene TNT Trotyl |
| Empirical formula | C7H5N3O6 |
| CAS Number | 118-96-7 |
| PubChem | 8376 |
| Short description | Pale, yellow, needle-shaped crystals |
| Characteristics | |
| Molar mass | 227.131 g/mol |
| Phase | Solid |
| Shock sensitivity | Insensitive |
| Friction sensitivity | Insensitive |
| Density | 1.654 g/cm³ |
| Explosive velocity | 6,900 m/s |
| RE factor | 1.00 |
| Melting Point | 80.35 °C |
| Boiling Point | 295 °C (Decomposition) |
| Vapor pressure | 5.7 Pa (81 °C) |
| Solubility |
|
| Safety References | |
| NFPA 704 | |
| R/S Statements |
R: 2-23/24/25-33-51/53 |
| TLV | 0.1 mg/m³ |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Trinitrotoluene or TNT (chemical formula C7H5N3O6) is a chemical explosive that has often been used in warfare. The purified material is a yellow, crystalline substance and is very toxic. Its formal name is 2-methyl-1,3,5-trinitrobenzene, in accordance with the nomenclature of the International Union of Pure and Applied Chemistry (IUPAC). First synthesized by Joseph Wilbrand in 1863, its large-scale production began in Germany in 1891. The explosive yield of TNT is considered the standard measure for the strength of bombs and other explosives (see TNT equivalent below).
History
TNT was first made in 1863 by German chemist Joseph Wilbrand, but its potential as an explosive was not recognized for several years, mainly because it was so hard to detonate and less powerful than other explosives. For example, in 1910, it was exempted from the UK's Explosives Act 1875, that is, not actually being considered an explosive for manufacturing and storage purposes. Among its advantages, however, is its ability to be safely melted using steam or hot water, allowing it to be poured molten into shell cases. (This is how Vietnamese fighters made their mines out of American shells during the Vietnam War.)
German armed forces adopted it as an artillery shell filling in 1902. During the First World War, the German Navy had the particular advantage of being able to detonate their TNT-filled armor-piercing shells after they had penetrated the armor of British capital ships. By contrast, the British lyddite-filled shells tended to explode as soon as they struck the German armor, thus expending much of their energy outside the ship. The British gradually started using it as replacement for lyddite in 1907.
Because of the insatiable demand for explosives during the Second World War, TNT was frequently mixed with 40 to 80 percent ammonium nitrate, producing an explosive called amatol. Although nearly as powerful as TNT (and much less expensive), amatol had the slight disadvantage of being hygroscopic (prone to absorbing moisture from the air). Another variation called minol, consisting of amatol mixed with about 20 percent aluminum powder, was used by the British in mines and depth charges. Although blocks of pure TNT are available in various sizes (such as 250 g, 500 g, and 1 kg) it is more commonly encountered in explosive blends that comprise a variable percentage of TNT plus other ingredients, such as torpex, tritonal, pentolite, and Composition B.
Synthesis
Trinitrotoluene is synthesized in a stepwise procedure. First, toluene is nitrated with a mixture of sulfuric and nitric acids. Even relatively low-concentration acid mixtures are capable of adding one or two nitro (NO2) groups to the toluene ring, producing mono- and dinitrotoluene. The nitro groups decrease the reactivity of the toluene drastically (because they are "electron-withdrawing" groups).
During the next stage, the mono- and dinitrotoluene are fully nitrated with a mixture of nitric acid and oleum (sulfuric acid with up to 60 percent dissolved sulfur trioxide (SO3)). This mixture is far more reactive and is capable of introducing the last (third) nitro group on the ring. The waste acid from this process is used for the first step of the reaction in industrial synthesis.
Characteristics
Trinitrotoluene takes the form of pale yellow, needle-shaped crystals and can be distilled in a vacuum. It is difficult to dissolve TNT in water; it is more soluble in ether, acetone, benzene, and pyridine. With its low melting point of 80.35 °C, TNT can be melted in steam and poured into containers. TNT is poisonous and skin contact can cause allergic reactions, causing the skin to turn a bright yellow-orange color.
- Water solubility: 130 mg/L at 20 °C
- Steam pressure at 20 °C: 150 to 600 Pa
- Detonation speed: 6700-7000 m/s 6900 m/s (density: 1,6 g/cm³)
- Lead block test: 300 ml/10 g
- Sensitivity to impact: 15 newton meter (N•m) (1.5 kilopound (kp)•meter (m))
- Friction sensitivity: to 353 N (36 kp) no reaction
Toxicity
Some military testing grounds are contaminated with TNT. Wastewater from munitions programs (including contaminated surface water and groundwater may be colored pink as a result of TNT and RDX contamination. Such contamination, called pinkwater, may be difficult and expensive to remedy.
TNT is quite toxic. It can also be absorbed through the skin, and will cause irritation and bright yellow staining. During the First World War, munition workers who handled the chemical found that their skin turned bright yellow. That led to the nickname "canary girls" or simply "canaries" to describe those workers. TNT would also eventually make ginger hair turn green. A 1916 British Government inquiry on female workers at the Royal Arsenal, Woolwich, found that 37 percent had severe pains due to loss of appetite, nausea, and constipation; 25 percent suffered from dermatitis; and 34 percent experienced changes in menstruation. Before respirators and protective grease applied to the skin were introduced, about 100 workers died from the disease.
People exposed to trinitrotoluene over a prolonged period tend to experience anemia and abnormal liver functions. Blood and liver effects, spleen enlargement and other harmful effects on the immune system have also been found in animals that ingested or breathed trinitrotoluene. There is evidence that TNT adversely affects male fertility, and TNT is listed as a possible human carcinogen. Consumption of TNT produces black urine.
TNT equivalent
TNT equivalent is a unit of energy commonly used to quantify large amounts of energy. One ton of TNT releases 4.184×109 joules upon explosion, therefore one kiloton of TNT is 4.184×1012 joules, and one megaton of TNT is 4.184×1015 joules.
A megaton is a large amount of energy. The first atomic bomb dropped on Hiroshima on August 6, 1945, exploded with the energy of about 20 kilotons of TNT (~8.4×1013 joules).
Current nuclear warheads in Russian and U.S. stockpiles range in yield from 100 kt to 20 Mt TNT equivalent. The largest bomb ever dropped, the Tsar Bomba, had a yield of about 50 Mt.
About one Mt equivalent exploded on the ground or slightly above ground creates a crater about 0.3 miles (0.5 km) in diameter and levels practically everything in a radius of a few miles or kilometers.
See also
ReferencesISBN links support NWE through referral fees
- Akhavan, J. 2004. The Chemistry of Explosives, 2nd edition. Cambridge, UK: The Royal Society of Chemistry. ISBN 0854046402
- Cooper, Paul W., and Stanley R. Kurowski. 1996. Introduction to the Technology of Explosives. New York, NY: Wiley-VCH. ISBN 047118635X
- Cooper, Paul W. 1996. Explosives Engineering. New York, NY: Wiley-VCH. ISBN 0471186368
- Meyer, Rudolf, Joseph Kohler, and Axel Homburg. 2002. Explosives, 5th revised edition. New York, NY: Wiley-VCH. ISBN 3527302670
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
