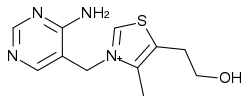
Thiamine
| Thiamine | |
|---|---|
| |
| Systematic name | Thiamine
|
| Molecular formula | C12H17N4OS
|
| Molecular mass | 266.4 g/mol |
| Density | x.xxx g/cm3 |
| Melting point | 248-250 °C (hydrochloride salt) |
| Boiling point | xx.x °C |
| CAS number | [59-43-8] [1] |
| SMILES | xxxx |
| Disclaimer and references | |
- For the similarly-spelled nucleic acid, see Thymine
Thiamine or thiamin, also known as vitamin B1, is one of the B vitamins, a group of chemically distinct, water-soluble vitamins that also includes riboflaven, niacin, pantothenic acid, pyridoxine, biotin, folic acid, and others. A colorless compound with chemical formula C12H17N4OS, thiamine's chemical structure contains a pyrimidine ring and a thiazole ring. It is insoluble in alcohol and decomposes if heated.
As a vitamin, thiamine is an organic (carbon-containing) nutrient obtained through the diet and is essential in small amounts for normal metabolic reactions in humans. Thiamine is integral to the complex coordination of the Krebs cycle, which is the main biochemical pathway to extract energy from glucose, amino acids, and fat (Podel 1999). Thiamine is essential for normal growth and development and helps to maintain proper functioning of the heart, nervous, and digestive systems. It serves as a co-enzyme in the pathway to synthesize NADPH and the pentose sugars deoxyribose and ribose, the later two of which are the sugars for DNA and RNA, respectively.
Since it is water-soluble, thiamine cannot be stored in the body; however, once absorbed, the vitamin is concentrated in muscle tissue. Balance and self-discipline in one's nutritional habits is necessary in order to ensure an adequate supply of the vitamins needed by the human body. Among good sources of thiamine are various vegetables, including legumes and green peas, as well as liver, nuts, and yeast. Beriberi is one well-known disease caused by a deficiency of thiamine.
Overview
Thiamine was first discovered in 1910 by Umetaro Suzuki in Japan when researching how rice bran cured patients of beriberi. He named it aberic acid. Suzuki did not determine its chemical composition, nor that it was an amine.
Thiamine was first crystallized by Jansen and Donath in 1926. (They named it aneurin, for antineuritic vitamin). Thiamine's chemical composition and synthesis was finally reported by Robert R. Williams in 1935. He also coined the name for it, thiamin.
There are four known natural thiamine phosphate derivatives—thiamine monophosphate (ThMP), thiamine diphosphate (ThDP), thiamine triphosphate (ThTP), and the recently discovered adenine thiamine triphopshate (AThTP).
In mammals, thiamine diphosphate (ThDP) or thiamine pyrophosphate (TPP) is a coenzyme for the enzymes pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, branched-chain alpha-keto acid dehydrogenase, 2-hydroxyphytanoyl-CoA lyase, and transketolase. The first two of these enzymes function in the metabolism of carbohydrates, while transketolase functions in the pentose phosphate pathway to synthesize NADPH and the pentose sugars deoxyribose and ribose. Deoxyribose is the sugar component of DNA, just as ribose serves that role in RNA (ribonucleic acid). ThDP is also the cofactor of pyruvate decarboxylase in yeast and of several bacterial enzymes.
In general, TPP functions as a cofactor for enzymes that catalyze the dehydrogenation (decarboxylation and subsequent conjugation to Coenzyme A) of alpha-keto acids. TPP is synthesized by the enzyme thiamine pyrophosphokinase, which requires free thiamine, magnesium, and adenosine triphosphate (ATP).
Thiamine triphosphate (ThTP) was long considered a specific neuroactive form of thiamine. However, recently it was shown that ThTP exists in bacteria, fungi, plants, and animals, suggesting a much more general cellular role. In particular, in Escherichia coli it seems to play a role in response to amino acid starvation.
Adenosine thiamine triphosphate (AThTP), or thiaminylated adenosine triphosphate, has recently been discovered in E. coli where it accumulates as a result of carbon starvation. In E. coli, AThTP may account for up to 20 percent of total thiamine. It also exists in lesser amounts in yeast, roots of higher plants, and animal tissues.
Nutrition, deficiency, and overdose
Thiamine is found naturally in the following foods, each of which contains at least 0.1 mg of the vitamin per 28-100g (1-3.5oz): green peas, spinach, liver, beef, pork, navy beans, nuts, pinto beans, soybeans, whole-grain and enriched cereals, breads, yeast, and legumes.
The aleurone layer of unpolished rice is a rich source.
The Reference Daily Intake (RDI), formerly called the Recommended Dietary Allowance (RDA) in most countries, is set at about 1.4 mg. However, studies on volunteers at daily doses of about 50 mg have shown an increase in mental acuity, including higher scores in terms of clear-headedness, better mood, and increased quickness on reaction-time tests (Podel 1999).
Systemic thiamine deficiency can lead to myriad problems, including neurodegeneration, wasting, and death. A lack of thiamine can be caused by malnutrition, alcoholism, a diet high in thiaminase-rich foods (raw freshwater fish, raw shellfish, ferns), and/or foods high in anti-thiamine factors, such as tea, coffee, betel nuts (Higdon 2002).
Well-known syndromes caused by thiamine deficiency include Wernicke-Korsakoff syndrome and beriberi—diseases also common with chronic alcoholism.
A positive diagnosis test for thiamine deficiency can be ascertained by measuring the activity of transketolase in erythrocytes. Thiamine can also be measured directly in whole blood following the conversion of thiamine to a fluorescent thiochrome derivative.
The only known cases of thiamine overdose occurred with thiamine injections. Thiamine injection may result in anaphylactic reactions.
Lonsdale et al. (2002) lead a successful pilot study on the treatment of autism spectrum children with thiamine. This work linking diet with autism is controversial.
Genetic diseases
Genetic diseases of thiamine transport are rare but serious. Thiamine Responsive Megaloblastic Anemia Syndrome (TRMA), also known as Rogers Syndrome, is a disorder where there is evidence it is caused by a defect in a thiamine transporter protein (McKusick et al. 2001). It is an early-onset, autosomal recessive disorder that is defined by the occurrence of megaloblastic anemia, as well as diabetes mellitus, and sensorineural deafness, and which responds in varying degrees to thiamine treatment (McKusick et al. 2004). It is traced to mutations in the gene SLC19A2 (McKusick et al. 2004), a high affinity thiamine transporter.
TRMA patients do not show signs of systemic thiamine deficiency, suggesting redundancy in the thiamine transport system. This has led to the discovery of a second high affinity thiamine transporter, SLC19A3 (Bocchini and McKusick 2005).
ReferencesISBN links support NWE through referral fees
- Bocchini, C. A., and V. A. McKusick. Solute Carrier Family 19 (Folate Transporter), Member 3; SLC19A3) Online Mendelian Interitence in Man National Center for Biotechnology Information and Johns Hopkins University, 2005. Retrieved August 14, 2007.
- Higdon, J. Thiamin Micronutrient Information Center. Oregon State University: Linus Pauling Institute, 2002. Retrieved August 14, 2007.
- McKusick, V. A., A. Hamosh, J. A. Phillips, and M. J. Wright. Thiamine-Responsive Megaloblastic Anemia Syndrome Online Mendelian Interitence in Man. National Center for Biotechnology Information and Johns Hopkins University, 2001. Retrieved August 14, 2007.
- McKusick, V. A., M. J. Wright, and G. E. Tiller. Solute Carrier Family 19 (thiamine transporter), Member 2; SLC19A2 Online Mendelian Interitence in Man National Center for Biotechnology Information and Johns Hopkins University, 2004. Retrieved August 14, 2007.
- Podel, R. N. Thiamine's mood-mending qualities. Nutrition Science News, 1999. Retrieved August 14, 2007.
- Siegel, George J., and Bernard W. Agranoff. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. Philadelphia: Lippincott Williams & Wilkins, 1999. ISBN 039751820X.
| Vitamins |
|---|
| All B vitamins | All D vitamins |
| Retinol (A) | Thiamine (B1) | Riboflavin (B2) | Niacin (B3) | Pantothenic acid (B5) | Pyridoxine (B6) | Biotin (B7) | Folic acid (B9) | Cyanocobalamin (B12) | Ascorbic acid (C) | Ergocalciferol (D2) | Cholecalciferol (D3) | Tocopherol (E) | Naphthoquinone (K) |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.