Vitamin E
| α-Tocopherol[1] | |
|---|---|

| |
| IUPAC name | (2R)-2,5,7,8-Tetramethyl-2-[(4R,8R)-4,8,12 -trimethyltridecyl]-3,4-dihydro-2H-chromen-6-ol |
| Identifiers | |
| CAS number | [] |
| EINECS number | |
| SMILES | CC(C)CCC[C@@H](C)CCC[C@@H](C)CCC [C@]1(C)CCc2c(C)c(O)c(C)c(C)c2O1 |
| InChI | InChI=1/C29H50O2/c1-20(2) 12-9-13-21(3)14-10- 15-22(4)16-11-18-29(8) 19-17-26-25(7)27(30) 23(5)24(6)28(26)31- 29/h20-22,30H,9- 19H2,1-8H3 |
| Properties | |
| Molecular formula | C29H50O2 |
| Molar mass | 430.69 g/mol |
| Density | 0.950 g/cm³ |
| Melting point |
2.5-3.5 °C |
| Boiling point |
200-220 °C at 0.1 mmHg |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Vitamin E is the generic descriptor for any of a group of several related fat-soluble organic compounds, tocopherols and tocotrienols, that act as vitamins with antioxidant properties. In particular, vitamin E is associated with α-tocopherol (also written as alpha-tocopherol). However, the term may apply to any tocopherol and tocotrienol derivatives exhibiting the biological effects of α-tocopherol, or it may apply as a collective term for the group.
As a vitamin, organic nutrients known as vitamin E are obtained through the diet and essential in small amounts for normal metabolic reactions. Vitamin E is important for its antioxidant properties in countering damage to cells from toxic oxygen produced as a by-product during metabolic processes. Vitamin helps to protect cell membranes from damage, and in particular the membranes of nerves. Other health benefits of vitamin E have been proposed, such as helping to combat heart disease, protect against certain cancers, and promote skin health, with diverse research findings.
There are numerous dietary sources of vitamin E, such as vegetable oils (palm oil, sunflower, corn, soybean, and olive oil), nuts, sunflower seeds, wheat germ, fish, whole grains, and green leafy vegetables. In addition to the harmony between human beings and their environment exhibited in the many sources of vitamin E, the complex coordination of the human body is seen in the function of vitamin E to counteract metabolic by-products. This harmony is broken down in the case of vitamin E deficiency. Although rare, because of the many dietary sources, vitamin E deficiency impacts the nervous system, resulting in neurological problems due to poor nerve conduction.
Overview
Vitamin E is a term that applies to a set of related tocopherols and tocotrienols. Tocopherols are organic compounds consisting of various methylated phenols. Tocotrienols are related compounds with the major structural difference from tocopherols being an unsaturated side chain that has three double bonds in its farnesyl isoprenoid tail. Tocopherols and tocotrienols are both fat-soluble oxidants. Any derivative of these main groups of compounds may exhibit vitamin E activity and be designated as vitamin E.
The terms vitamin E and tocopherol are sometimes used interchangeably but they are not synonymous. IUPAC (1981) states:
The term vitamin E should be used as the generic descriptor for all tocol and tocotrienol derivatives exhibiting qualitatively the biological activity of α-tocopherol. This term should be used in derived terms such as vitamin E deficiency, vitamin E activity, vitamin E antagonist.
The term tocopherol(s) should be used as a generic descriptor for all mono, di, and trimethyltocols. Thus, this term is not synonymous with the term vitamin E.
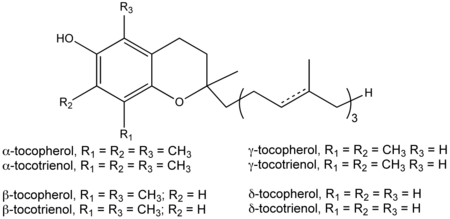
Generally, eight basic forms of vitamin E are recognized, four tocopherols and four tocotrienols (Herrera and Barbas 2001; Packer et al. 2001). There are four isomers of tocopherols: α- (or alpha-), β- (beta-), γ- (gamma-) and δ- (delta-) tocopherols. Likewise, there are four isomers of tocotrienols: α-, β-, γ-, and δ-tocopherols. Each of the four isomers contain different number of methyl groups on the chromanol ring.
Of these, α-tocopherol (also written as alpha-tocopherol) has been most studied as it has the highest bioavailability, with the body preferentially absorbing and using this form (Brigelius-Flohé and Traber 1999). It is the form most commonly associated with vitamin E and used in vitamin E supplements. It has been claimed that α-tocopherol is the most important lipid-soluble antioxidant, and that it protects cell membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction (Traber and Atkinson 2007). This would remove the free radical intermediates and prevent the oxidation reaction from continuing. The oxidized α-tocopheroxyl radicals produced in this process may be recycled back to the active reduced form through reduction by other antioxidants, such as ascorbate, retinol, or ubiquinol (Wang and Quinn 1999). The compound α-tocopherol is a common form of tocopherol added to food products.
The functions of the other forms of vitamin E are less well-studied, although γ-tocopherol (also written as gamma-tocopherol) is a nucleophile that may react with electrophilic mutagens (Brigelius-Flohé and Traber 1999) and the tocotrienols may have specialized roles in protecting neurons from damage (Sen et al. 2006), cancer prevention (Malafa 2008), and cholesterol reduction (Das et al. 2008). However, the roles and importance of all of the various forms of vitamin E are presently unclear (Brigelius-Flohé and Davies 2007; Traber and Atkinson 2007; Atkinson et al. 2007), and it has even been suggested that the most important function of vitamin E is as a signaling molecule, and that it has no significant role in antioxidant metabolism (Azzi 2007).
Most studies about vitamin E have supplemented using only the synthetic alpha-tocopherol, but doing so leads to reduced serum gamma- and delta-tocopherol concentrations. Moreover, a 2007 clinical study involving synthetic alpha-tocopherol concluded that supplementation did not reduce the risk of major cardiovascular events in middle aged and older men (Sesso et al. 2008).
Forms and structures
Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromanol ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals and a hydrophobic side chain, which allows for penetration into biological membranes. Both the tocopherols and tocotrienols occur in alpha, beta, gamma, and delta forms, determined by the number of methyl groups on the chromanol ring. Each form has slightly different biological activity (Burton and Ingold 1981).
As a food additive, tocopherol is labeled with these E numbers: E307 (α-tocopherol), E308 (γ-tocopherol), and E309 (δ-tocopherol).
Alpha-tocopherol
Alpha-tocopherol is the form of vitamin E that is preferentially absorbed and accumulated in humans (Rigotti 2007). The measurement of "vitamin E" activity in international units (IU) was based on fertility enhancement by the prevention of spontaneous abortions in pregnant rats relative to alpha-tocopherol.
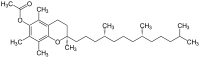
There are three stereocenters in alpha-tocopherol, so this is a chiral molecule (Jensen and Lauridsen 2007). The eight stereoisomers of alpha-tocopherol differ in the arrangement of groups around these stereocenters. In the image of RRR-alpha-tocopherol below, all three stereocenters are in the R form. However, if the middle of the three stereocenters were changed (so the hydrogen was now pointing down and the methyl group pointing up), this would become the structure of RSR-alpha-tocopherol. RSR-alpha-tocopherol and RRR-alpha-tocopherol are mirror-images of each other. These stereoisomers can also be named in an alternative older nomenclature, where the stereocenters are either in the d or l form (Brigelius-Flohé and Traber 1999).
One IU of vitamin E is the biological equivalent of about 0.667 milligrams (2/3 milligrams exactly) of RRR-alpha-tocopherol (formerly named d-alpha-tocopherol or sometimes ddd-alpha-tocopherol). One IU is also defined as 1 milligram of an equal mix of the eight stereoisomers, which is a racemic mixture called all-rac-alpha-tocopheryl acetate. This mix of stereoisomers is often called dl-alpha-tocopheryl acetate, even though it is more precisely dl,dl,dl-alpha-tocopheryl acetate. However, 1 IU of this racemic mixture is not now considered equivalent to 1 IU of natural (RRR) α-tocopherol, and the Institute of Medicine and the USDA now convert IU's of the racemic mixture to milligrams of equivalent RRR using 1 IU racemic mixture = 0.45 "milligrams α-tocopherol" (USDA 2008).
Other R, R, R tocopherol
The other R, R, R tocopherol vitamins are slowly being recognized as research begins to elucidate their additional roles in the human body. Many naturopathic and orthomolecular medicine advocates suggest that vitamin E supplements contain at least 20 percent by weight of the other natural vitamin E isomers.
Tocotrienols
Tocotrienols, with four d- isomers, although less commonly known, also belong to the vitamin E family. The four tocotrienols have structures corresponding to the four tocopherols, except with an unsaturated bond in each of the three isoprene units that form the hydrocarbon tail, whereas tocopherols have a saturated phytyl tail. Tocotrienol has been subject to fewer clinical studies and seen less research as compared to tocopherol. However there is growing interest in the potential health benefits of these compounds (Sen et al. 2006).
History
During feeding experiments with rats Herbert McLean Evans concluded, in 1922, that besides vitamins B and C, an unknown vitamin existed (Evans and Bishop 1922). Although every other known nutritient was present, the rats were not fertile. This condition could be changed by additional feeding with wheat germ. It took several years, until 1936, when the substance was isolated from wheat germ and the formula C29H50O2 was determined. Evans also found that the compound reacted like an alcohol and concluded that one of the oxygen atoms was part of an OH (hydroxyl) group. The name tocopherol by Evans from Greek words meaning "to bear young" or "to carry a pregnancy" (“τοκος” meaning "birth," and “φορειν” meaning "to bear or carry")," with the ending "-ol" signifying its status as a chemical alcohol (Evans et al. 1936).
The structure natural a-tocopherol, the most potent natural source of vitamin E activity, was elucidated shortly thereafter, in 1938 (Fernholz 1938).
Importance
Function
Vitamin A has the function in the human body of preventing that natural and continual process of deterioration of body tissues, a deterioration that is provoked by a number of causes including toxic oxygen. As the body metabolizes atmospheric oxygen, toxic oxygen is produced in the body through such by-products as hydrogen peroxide, superoxide, and hypochlorite. Hypochlorite is a natural product, produced by immune system cells (and is a component of bleach). Toxic oxygen can damage cells and tissues of the body, such as cell membranes. Vitamin E appears to serve the body by protecting membranes from toxic oxygen damage. (Vitamin C protects the interior, watery regions of cells from toxic oxygen damage.) The most sensitive membranes to toxic oxygen appear to the the membranes of nerves and thus vitamin E deficiency damages the nervous system (Brody 2004).
Deficiency
Vitamin E deficiency is very rare but can strike people with diseases that prevent absorption of dietary fats and fat soluble nutrients (Brody 2004). Vitamin E deficiency causes neurological problems due to poor nerve conduction. These include neuromuscular problems such as spinocerebellar ataxia and myopathies (Brigelius-Flohé and Traber 1999). Deficiency can also cause anemia, due to oxidative damage to red blood cells.
Dietary sources
In foods consumed by people, the most abundant sources of vitamin E are vegetable oils such as palm oil, sunflower, corn, soybean, and olive oil. Nuts, sunflower seeds, seabuckthorn berries, kiwifruit, and wheat germ are also good sources. Other sources of vitamin E are whole grains, fish, peanut butter, goats milk, and green leafy vegetables. Fortified breakfast cereals are also an important source of vitamin E in the United States. Although originally extracted from wheat germ oil, most natural vitamin E supplements are now derived from vegetable oils, usually soybean oil.
The content of Vitamin E for rich sources follows (Bauernfeind 1980):
- Wheat germ oil (215.4 mg/100 g)
- Sunflower oil (55.8 mg/100 g)
- Almond oil (39.2 mg/100 g)
- Hazelnut (26.0 mg/100 g)
- Walnut oil (20.0 mg/100 g)
- Peanut oil (17.2 mg/100 g)
- Olive oil (12.0 mg/100 g)
- Peanut (9.0 mg/100 g)
- Pollard (2.4 mg/100 g)
- Corn (2.0 mg/100 g)
- Asparagus (1.5 mg/100 g)
- Oats (1.5 mg/100 g)
- Chestnut (1.2 mg/100 g)
- Coconut (1.0 mg/100 g)
- Tomatoes (0.9 mg/100 g)
- Carrots (0.6 mg/100 g)
- Goat's milk (0.1 mg/100ml)
Recommended amounts
The U.S. Dietary Reference Intake (DRI) Recommended Daily Amount (RDA) for a 25 year old male for Vitamin E is 15 milligrams(mg)/day. The DRI for vitamin E is based on the alpha-tocopherol form because it is the most active form as originally tested.
Results of two national surveys, the National Health and Nutrition Examination Survey (NHANES III 1988-91) and the Continuing Survey of Food Intakes of Individuals (1994 CSFII) indicated that the dietary intakes of most Americans do not provide the recommended amounts of vitamin E. However, a 2000 Institute of Medicine (IOM) report on vitamin E states that intake estimates of vitamin E may be low because energy and fat intake is often under reported in national surveys and because the kind and amount of fat added during cooking is often not known. The IOM states that most North American adults get enough vitamin E from their normal diets to meet current recommendations. However, they do caution individuals who consume low fat diets because vegetable oils are such a good dietary source of vitamin E. Vitamin E supplements are absorbed best when taken with meals (Iuliano et al. 2001).
Because vitamin E can act as an anticoagulant and may increase the risk of bleeding problems, many agencies have set an upper tolerable intake level (UL) for vitamin E at 1,000 mg (1,500 IU) per day (NIH-ODS).
Supplements
Commercial vitamin E supplements can be classified into several distinct categories:
- Fully synthetic vitamin E, "dl-alpha-tocopherol," the most inexpensive, most commonly sold supplement form usually as the acetate ester
- Semi-synthetic "natural source" vitamin E esters, the "natural source" forms used in tablets and multiple vitamins. These are highly fractionated d-alpha tocopherol or its esters, often made by synthetic methylation of gamma and beta d,d,d tocopherol vitamers extracted from plant oils.
- Less fractionated "natural mixed tocopherols" and high d-gamma-tocopherol fraction supplements
Synthetic all-racemic
Synthetic vitamin E derived from petroleum products is manufactured as all-racemic alpha tocopheryl acetate with a mixture of eight stereoisomers. In this mixture, one alpha-tocopherol molecule in eight molecules are in the form of RRR-alpha-tocopherol (12.5 percent of the total) (Weiser et al. 1996).
The 8-isomer all-rac vitamin E is always marked on labels simply as dl-tocopherol or dl-tocopheryl acetate, even though it is (if fully written out) actually dl,dl,dl-tocopherol. The present largest manufacturers of this type are DSM and BASF.
(An earlier semisynthetic vitamin E actually contained 50 percent d,d,d-alpha tocopherol moiety and 50 percent l,d,d-alpha-tocopherol moiety, as synthesized by an earlier process, which started with a plant sterol intermediate with the correct chirality in the tail, and thus resulted in a racemic mixture at only one chiral center. This form, known as 2-ambo tocopherol, is no longer made.)
Natural alpha-tocopherol is the RRR-alpha (or ddd-alpha) form. The synthetic dl,dl,dl-alpha ("dl-alpha") form is not as active as the natural ddd-alpha ("d-alpha") tocopherol form. This is mainly due to reduced vitamin activity of the 4 possible stereoisomers which are represented by the l or S enantiomer at the first stereocenter (an S or l configuration between the chromanol ring and the tail, that is, the SRR, SRS, SSR, and SSS stereoisomers) (Jensen and Lauridsen 2007). Unnatural 2R stereoisomers with natural R configuration at this stereocenter, but S at the other centers in the tail (RSR, RRS), appear to retain substantial RRR vitamin activity because they are recognized by the alpha-tocopherol transport protein, and thus maintained in the plasma, where the other four stereoisomers are not. Thus, the synthetic all-rac-α-tocopherol probably has only about half the vitamin activity of RRR-alpha-tocopherol in humans, even though the ratio of activities of the 8 stereoisomer racemic mixture to the natural vitamin is 1 to 1.36 in the rat pregnancy model.[2]
Although it is clear that mixtures of stereoisomers are not as active as the natural RRR-alpha-tocopherol form, in the ratios discussed above, specific information on any side effects of the seven synthetic vitamin E stereoisomers is not readily available. Some naturopathic and orthomolecular medicine advocates have held that none of the other stereoisomers of vitamin E have merit for cancer, circulatory and heart diseases, but hold this opinion without being able to point to definitive studies of the matter.
Esters
Manufacturers also commonly convert the phenol form of the vitamins (with a free hydroxyl group) to esters, using acetic or succinic acid. These tocopheryl esters are more stable and are easy to use in vitamin supplements. Alpha tocopheryl esters are de-esterified in the gut and then absorbed as the free tocopherol (Mathias et al. 1981a; Ajandouz et al. 2006).
An initial study in humans saw large variability between different people's absorption of all these forms of vitamin E, with no statistically-significant differences seen between tocopheryl esters and the free tocopherol (Horwitt et al. 1984). Later studies saw no difference between the rate of absorption of these forms of vitamin E and found that tocopheryl esters and free tocopherol had the same bioavailability (Cheeseman et al. 1995; Burton et al. 1988). The esterase activity responsible for releasing the free tocopherol may be reduced in children with cystic fibrosis (Mathias et al. 1981b).
Tocopheryl nicotinate and tocopheryl linolate esters are used in cosmetics and some pharmaceuticals.
Mixed tocopherols
"Mixed tocopherols" in the United States contain at least 20 percent w/w (weight/weight) of other natural R, R,R- tocopherols; for instance, R, R,R-alpha-tocopherol content plus at least 25 percent R, R,R-beta-, R, R,R-gamma-, R, R,R-delta-tocopherols.
Some brands may contain 200 percent w/w or more of the other tocopherols and measurable tocotrienols. Some mixed tocopherols with higher gamma-tocopherol content are marketed as "High Gamma-Tocopherol." The label should report each component in milligrams, except R, R,R-alpha-tocopherol may still be reported in IU. Mixed tocopherols can also be found in other nutritional supplements.
Safety
"Megadoses" of vitamin E are not recommended by many government agencies, due to a possible increased risk of bleeding. A meta-analysis by Miller et al. (2005) found that high-dosage vitamin E supplements may increase all-cause mortality. "High dose" vitamin E esters (>400 units/day) were also associated with an increased risk in all-cause mortality of 39 per 10,000 persons, and a statistically significant relation existed between dose and mortality, with increased risk at doses exceeding 150 units per day. These trials included synthetic beta-carotene and other confounders. However, the Miller study was criticized as "seriously flawed" in a Journal of the American Nutraceutical Association article by Houston (2005).
A review of a number of randomized controlled trials in the scientific literature by the Cochrane Collaboration, published in JAMA in 2007, also found an increase in mortality, of 4 percent (Relative Risk 1.04, 95 percent confidence interval 1.01-1.07), or 400 per 10,000 persons (Bjelakovic et al. 2007).
Other health uses
As noted above, vitamin E appears to function as antioxidant that helps in the maintenance of cell membranes, as well as helping to protect vitamin A and vitamin C. Vitamin E helps to counteract toxic oxygen products that can damage parts of the body, such as the cell membranes, and in particular the membranes of nerves.
However, other uses for vitamin E have been suggested and tested.
Conventional medical studies on vitamin E, as of 2006 and as below, use either a synthetic all-racemic ("d, l-") alpha tocopheryl ester (acetate or succinate) or a semi-synthetic d-alpha tocopheryl ester (acetate or succinate). Proponents of megavitamin, orthomolecular and naturally based therapies have advocated, for the last two thirds of a century, and have used the natural tocopherols, often mixed tocopherols with an additional 25 to 200 percent w/w (weight/weight) d-beta-, d-gamma- (Jiang et al. 2001; Gaziano 2004), and d-delta-tocopherol. Based on various clinical, experimental, patent, and individual data, natural health proponents have long held (Bailey 1964; Walker 1992) that the other poorly studied tocopherols, especially the abundant d-gamma-tocopherol (MacWilliam 2006), in combination with other antioxidants such as selenium, coQ10, vitamin C, vitamin K2, mixed carotenoids, and lipoic acid, provide unique biochemical benefits (Houston 2005).
The methodology, interpretation and reporting of conventional vitamin E studies have even become contentious within conventional medicine circles (Carter 2005).
Topical use
Vitamin E is widely used in industry as an inexpensive antioxidant (namely for cosmetics and foods). Vitamin E containing products are commonly used in the belief that vitamin E is good for the skin; many cosmetics include it, often labeled as tocopherol acetate, tocopheryl linoleate, or tocopheryl nicotinate. Individuals can still experience allergic reactions to some tocopheryl esters or develop a rash and hives that may spread over the entire body from the use of topical products with alpha tocopheryl esters (Dermweb 1996).
Reduce scarring
On the basis of limited research (Palmieri et al. 1995), topical applications of Vitamin E are often claimed by manufacturers of skin creams and lotions to play a role in encouraging skin healing and reducing scarring after injuries, such as burns. However, evidence of a benefit of silicon gel sheeting, with or without added Vitamin E, is limited by the poor quality of the research (O'Brien and Pandit 2006). One study found that it did not improve or worsen the cosmetic appearance in 90 percent of patients, with a third developing contact dermatitis (Baumann and Spencer 1999).
During pregnancy
Recent studies into the use of both vitamin C and the single isomer vitamin E esters as possible help in preventing oxidative stress leading to pre-eclampsia has failed to show significant benefits (Rumbold et al. 2006), but did increase the rate of babies born with a low birthweight in one study (Poston et al. 2006).
Heart disease
Preliminary research has led to a widely held belief that vitamin E may help prevent or delay coronary heart disease, but larger controlled studies have not confirmed such a benefit (Sesso et al. 2008).
Many researchers advance the belief that oxidative modification of LDL-cholesterol (sometimes called "bad" cholesterol) promotes blockages in coronary arteries that may lead to atherosclerosis and heart attacks. Thus, it is held that vitamin E may help prevent or delay coronary heart disease by limiting the oxidation of LDL-cholesterol. Vitamin E also is proposed to help prevent the formation of blood clots, which could lead to a heart attack.
Observational studies have associated lower rates of heart disease with higher vitamin E intake. A study of approximately 90,000 nurses suggested that the incidence of heart disease was 30 to 40 percent lower among nurses with the highest intake of vitamin E from diet and supplements. The range of intakes from both diet and supplements in this group was 21.6 to 1,000 IU (32 to 1,500 mg), with the median intake being 208 IU (139 mg). A 1994 review of 5,133 Finnish men and women aged 30-69 years suggested that increased dietary intake of vitamin E was associated with decreased mortality (death) from heart disease.
But even though these observations are promising, randomized clinical trials have consistently shown lack of benefit to the role of vitamin E supplements in heart disease. The Heart Outcomes Prevention Evaluation (HOPE) Study followed almost 10,000 patients for 4.5 years who were at high risk for heart attack or stroke. In this intervention study, the subjects who received 265 mg (400) IU of vitamin E daily did not experience significantly fewer cardiovascular events or hospitalizations for heart failure or chest pain when compared to those who received a sugar pill. The researchers suggested that it is unlikely that the vitamin E supplement provided any protection against cardiovascular disease in the HOPE study. This study is continuing, to determine whether a longer duration of intervention with vitamin E supplements will provide any protection against cardiovascular disease.
Furthermore, meta analysis of several trials of antioxidants, including vitamin E, have not shown any benefit to vitamin E supplementation for preventing coronary heart disease (Vivekananthan et al. 2003). One study suggested that vitamin E (as alpha-tocopherol only) supplementation may increase the risk for heart failure (Lonn et al. 2005). Supplementing alpha-tocopherol without gamma-tocopherol is known to lead to reduced serum gamma- and delta-tocopherol concentrations (Huang and Appel 2003).
Cancer
Antioxidants such as vitamin E help protect against the damaging effects of free radicals, which may contribute to the development of chronic diseases such as cancer. Vitamin E also may block the formation of nitrosamines, which are carcinogens formed in the stomach from nitrites consumed in the diet. It also may protect against the development of cancers by enhancing immune function. To date, human trials and surveys that have tried to associate vitamin E with incidence of cancer remain generally inconclusive.
Some evidence associates higher intake of vitamin E with a decreased incidence of prostate cancer and breast cancer. Some studies correlate additional cofactors, such as specific vitamin E isomers, for example, gamma-tocopherol and other nutrients such as selenium, with dramatic risk reductions in prostate cancer (Helzlsouer et al. 2000). There has been speculation that vitamin E coupled with selenium may reduce the risk of prostate cancer (ACS 2008) by 30 percent (NCI 2008a). However, the Selenium and Vitamin E Cancer Prevention Trial, ("SELECT"), run from 2004 to 2008, found that vitamin E, whether taken alone or in combination with selenium, did not prevent prostate cancer (NCI 2008b). The SELECT study was discontinued after independent reviewers determined that there was no benefit to the 35,000 men who were the subject of the study (ACS 2008). Likewise, an examination of the effect of dietary factors, including vitamin E, on incidence of postmenopausal breast cancer in over 18,000 women from New York State did not associate a greater vitamin E intake with a reduced risk of developing breast cancer. A study of the effect on lung cancer in smokers also showed no benefit (BCCPSG 1994).
Recent studies have found that increased intake of vitamin E, especially among smokers, may be responsible for an increase in the incidence of lung cancer, with one study finding an increase in the incidence of lung cancer by 7 percent for each 100IU of vitamin E taken daily (Mahabir et al. 2008; Cancer Research UK 2008; NIP 2008;).
Cataracts
A cataract is a condition of clouding of the tissue of the lens of the eye. Cataracts increase the risk of disability and blindness in aging adults. Antioxidants are being studied to determine whether they can help prevent or delay cataract growth. Observational studies have found that lens clarity, which is used to diagnose cataracts, was better in regular users of vitamin E supplements and in persons with higher blood levels of vitamin E. A controlled trial of high doses of vitamins C and E and beta carotene found no effect on the risk of developing cataracts (AREDS 2001a). Similarly, a trial using vitamin E alone found that vitamin E supplementation produced no change in the risk of developing cataracts or the rate of progression of existing cataracts (McNeil et al. 2004).
Age-related macular degeneration (AMD) is the leading cause of visual impairment and blindness in the United States and the developed world among people 65 years and older. There has been research that suggests vitamin E alone does not attenuate the development or progression of AMD (Taylor et al. 2002).
However, studies focusing on efficacy of Vitamin E combined with other antioxidants, like zinc and vitamin C, indicate a protective effect against the onset and progression of AMD (AREDS 2001b; van Leeuwen et al. 2005; Moriarty-Craige et al. 2005).
Glaucoma
A 2007 study published in the European Journal of Ophthalmology found that, along with other treatments for glaucoma, adding alpha-tocopherol appeared to help protect the retina from glaucomatous damage. Groups receiving 300 mg and 600 mg per day of alpha-tocopherol, delivered orally, showed statistically significant decreases in the resistivity index in the posterior ciliary arteries and in the pulsatility index in the ophthalmic arteries, after six and twelve months of therapy. Alpha-tocopherol-treated patients also had significantly lower differences in mean visual field deviations (Engin et al. 2007).
Alzheimer's disease
Alzheimer's disease is a wasting disease of the brain. As oxidative stress may be involved in the pathogenesis of Alzheimer's, tocopherols have been tested as both a means of preventing and treating this disease. The results of these studies have been mixed, with some research suggesting that high levels of vitamin E in the diet may reduce the risk of Alzheimer's, while other studies found no such link (Frank and Gupta 2005). Similarly, studies on whether tocopherols could slow the progression of Alzheimer's have also been contradictory, with the Alzheimer’s Disease Cooperative Study suggesting that vitamin E supplementation might be beneficial, but a later trial finding no clinical benefit (Ricciarelli et al. 2007). Due to this contradictory and confusing evidence, vitamin E or tocopherol supplements are not currently recommended for treating or preventing Alzheimer's disease (Boothby and Doering 2005).
Parkinson's disease
In May 2005, The Lancet Neurology published a study suggesting that vitamin E may help protect against Parkinson's disease (Etminan et al. 2005). Individuals with moderate to high intakes of dietary vitamin E were found to have a lower risk of Parkinson's. No conclusion could be made from this trial about whether supplemental vitamin E has the same effect, however (BBC 2005). Other trials have tested if giving vitamin E supplements reduce the risk of Parkinson's disease, or if they can slow the progression of the disease. In a 1998 study, vitamin E supplements had no effect on the rate of progression of this disease (Shoulson 1998).
Notes
- ↑ Merck Index, 11th Edition, 9931.
- ↑ "Taken together, these data indicate that of the eight stereoisomers (RRR, RSR, RRS, RSS, SRR, SSR, SRS, SSS) in all-rac-α-tocopherol, only the four 2R-forms (RRR, RSR, RSS, RRS) are recognized by α-TTP and maintained in the plasma. Indeed, the Food and Nutrition Board (Food and Nutrition Board and Institute of Medicine, 2000) has defined that only α-tocopherol, specifically the 2R-forms of α-tocopherol, can fulfill the human requirement for vitamin E. Thus, all-rac-α-tocopherol has only half the activity of RRR-α-tocopherol." Taken from C. Lauridsen, H. Engel, A. M. Craig and M. G. Traber, "Relative bioactivity of dietary RRR- and all-rac-alpha-tocopheryl acetates in swine assessed with deuterium-labeled vitamin E1," J Anim Sci 80(2002):702-707. Retrieved January 16, 2009.
ReferencesISBN links support NWE through referral fees
- Age-Related Eye Disease Study Research Group (AREDS). 2001a. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch. Ophthalmol. 119(10): 1439–52. PMID 11594943. Retrieved January 16, 2009.
- Age-Related Eye Disease Study Research Group (AREDS). 2001b. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 119(10): 1417–36. PMID 11594942. Retrieved January 16, 2009.
- Ajandouz el, H., C. Castan, S. Jakob, and A. Puigserver. 2006. A fast, sensitive HPLC method for the determination of esterase activity on alpha-tocopheryl acetate. J Chromatogr Sci 44(10): 631–3. PMID 17254374. Retrieved January 16, 2009.
- American Cancer Society. 2008. Vitamin E. Cancer.org. Retrieved January 16, 2009.
- Atkinson, J., R. F. Epand, and R. M. Epand. 2007. Tocopherols and tocotrienols in membranes: A critical review. Free Radic. Biol. Med. 44(5): 739–764. PMID 18160049. Retrieved January 16, 2009.
- Bailey, H. 1964. Vitamin E: Your Key to a Healthy Heart. Chilton Books.
- Bauernfeind, J. in L. J. Machlin, ed., Vitamin E: A Comprehensive Treatise. New York: M. Dekker. ISBN 0824768426.
- Baumann, L. S., and J. Spencer. 1999. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg 25(4): 311–5. PMID 10417589.
- BBC. 2005. Vitamin E cuts Parkinson's risk. BBC May 19, 2005. Retrieved January 19, 2009.
- Beta Carotene Cancer Prevention Study Group (BCCPSG). 1994. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 330(15): 1029–35. PMID 8127329. Retrieved January 16, 2009.
- Boothby, L. A., and P. L. Doering. 2005. Vitamin C and vitamin E for Alzheimer's disease. Ann Pharmacother 39(12): 2073–80. PMID 16227450.
- Brigelius-Flohé, R., and K. J. Davies. 2007. Is vitamin E an antioxidant, a regulator of signal transduction and gene expression, or a 'junk' food? Comments on the two accompanying papers: "Molecular mechanism of alpha-tocopherol action" by A. Azzi and "Vitamin E, antioxidant and nothing more" by M. Traber and J. Atkinson. Free Radic. Biol. Med. 43(1): 2–3. PMID 17561087. Retrieved January 16, 2009.
- Brigelius-Flohé, R., and M. G. Traber. 1999. Vitamin E: function and metabolism. FASEB J. 13(10): 1145–55. PMID 10385606. Retrieved January 16, 2009.
- Brody, T. 2004. Vitamin E deficiency. In J. L. Longe, The Gale Encyclopedia of Medicine, 2nd ed. Detroit: Gale Group/Thomson Learning. ISBN 0787654949.
- Burton, G. W., and K. U. Ingold. 1981. Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 103: 6472-6477.
- Burton, G. W., K. U. Ingold, D. O. Foster, et al. 1988. Comparison of free alpha-tocopherol and alpha-tocopheryl acetate as sources of vitamin E in rats and humans. Lipids 23(9): 834–40. PMID 3185118. Retrieved January 16, 2009.
- Bjelakovic, G., D. Nikolova, L. L. Gluud, et al. 2007. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 297:842. PMID 17327526. Retrieved January 16, 2009.
- Cancer Research UK. 2008. Jury still out over vitamin E supplements and increased lung cancer risk. Cancer Research UK. Retrieved January 16, 2008.
- Carter, T. 2005. Responses and Comments: High-Dosage Vitamin E Supplementation and All-Cause Mortality. Ann Intern Med. 143(2):155. Retrieved January 16, 2008.
- Cheeseman, K. H., A. E. Holley, F. J. Kelly, M. Wasil, L. Hughes, and G. Burton. 1995. Biokinetics in humans of RRR-alpha-tocopherol: The free phenol, acetate ester, and succinate ester forms of vitamin E. Free Radic. Biol. Med. 19(5): 591–8. PMID 8529918. Retrieved January 16, 2008.
- Das, S., I. Lekli, et al. 2008. Cardioprotection with palm oil tocotrienols: Comparision of different isomers. Am J Physiol Heart Circ Physiol 294(2): 970-978. Retrieved January 16, 2009.
- Dermweb. 1996. Topical vitamin E formulations: Not always benign. Skin Therapy Letter 1(3). Retrieved January 16, 2009.
- Engin, K. N., G. Engin, H. Kucuksahin, M. Oncu, G. Engin, and B. Guvener. 2007. Clinical evaluation of the neuroprotective effect of alpha-tocopherol against glaucomatous damage. European Journal of Ophthalmology 17(4): 528–33. PMID 17671926. Retrieved January 16, 2009.
- Etminan, M., S. S. Gill, and A. Samii. 2005. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson's disease: A meta-analysis. Lancet Neurol 4(6): 362–5. PMID 15907740. Retrieved January 16, 2009.
- Evans H. M., and K. S. Bishop. 1922. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 56(1458): 650–651.
- Evans H. M., O. H. Emerson, and G. A. Emerson. 1936. The isolation from wheat germ oil of an alcohol, a-tocopherol, having the properties of vitamin E. Journal of Biological Chemistry 113(1): 319–332. Retrieved January 16, 2009.
- Fernholz, E. 1938. On the constitution of α-Tocopherol. Journal of the American Chemical Society 60(1): 700–705. Retrieved January 16, 2009.
- Frank, B., and S. Gupta. 2005. A review of antioxidants and Alzheimer's disease. Ann Clin Psychiatry 17(4): 269–86. PMID 16402761. Retrieved January 16, 2009.
- Gaziano, J. M. 2004. Vitamin E and cardiovascular disease: Observational studies. Ann. N.Y. Acad. Sci. 1031: 280–291.
- Helzlsouer, K., H. Huang, A. Alberg, S. Hoffman, A. Burke, E. Norkus, J. Morris, and G. Comstock. 2000. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst 92(24): 2018–23. PMID 11121464. Retrieved January 16, 2009.
- Herrera, E., and C. Barbas. 2001. Vitamin E: Action, metabolism and perspectives. J Physiol Biochem 57(2): 43–56. PMID 11579997. Retrieved January 16, 2009.
- Horwitt, M., W. Elliott, P. Kanjananggulpan, and C. Fitch. 1984. Serum concentrations of alpha-tocopherol after ingestion of various vitamin E preparations. Am J Clin Nutr 40(2): 240–5. PMID 6465056. Retrieved January 16, 2009.
- Houston, M. 2005. Meta-analysis, metaphysics and mythology: Scientific and clinical perspective on the controversies regarding vitamin E for the prevention and treatment of disease in humans JANA 8(1). Retrieved January 16, 2009. (pdf)
- Huang, H. Y., and L. J. Appel. 2003. Supplementation of diets with alpha-tocopherol reduces serum concentrations of gamma- and delta-tocopherol in humans. J. Nutr. 133(10): 3137–40. PMID 14519797. Retrieved January 16, 2009.
- International Union of Pure and Applied Chemistry (IUPAC) and the International Union of Bichemistry and Molecular Biology (IUB). 1981. Nomenclature of Tocopherols and Related Compounds (Recommendations 1981). IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Retrieved January 16, 2009.
- Iuliano, L., F. Micheletta, M. Maranghi, G. Frati, U. Diczfalusy, and F. Violi. 2001. Bioavailability of vitamin E as function of food intake in healthy subjects. Arteriosclerosis, Thrombosis, and Vascular Biology 21: e34–e37. PMID 11597949. Retrieved January 16, 2009.
- Jensen, S. K., and C. Lauridsen. 2007. Alpha-tocopherol stereoisomers. Vitam. Horm. 76: 281–308. PMID 17628178. Retrieved January 16, 2009.
- Jiang, Q., S. Christen, M. K. Shigenaga, and B. N. Ames. 2001. Gamma tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr 74: 714-22.
- Lauridsen, C., H. Engel, A. M. Craig, and M. G. Traber. 2001. Relative bioactivity of dietary RRR- and all-rac-alpha-tocopheryl acetates in swine assessed with deuterium-labeled vitamin E1. J Anim Sci 80: 702-707. Retrieved January 16, 2009.
- Lonn, E., J. Bosch, S. Yusuf, P. Sheridan, J. Pogue, J. Arnold, C. Ross, A. Arnold, P. Sleight, J. Probstfield, and G. Dagenais. 2005. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA 293(11): 1338–47. PMID 15769967. Retrieved January 16, 2009.
- MacWilliam, L. 2006. What makes gamma tocopherol superior to alpha tocopherol. Life Extension Magazine April 2006. Retrieved January 16, 2009.
- Mahabir, S., K. Schendel, Y. Q. Dong, S. L. Barrera, M. R. Spitz, and M. R. Forman. Dietary alpha-, beta-, gamma- and delta-tocopherols in lung cancer risk. International Journal of Cancer 123: 1173–1180.
- Malafa, M. P. 2008. New insights and gains in pancreatic cancer. Cancer Control 15(4): 276-277. Retrieved January 16, 2009.
- Mathias, P. M., J. T. Harries, T. J. Peters, and D. P. Muller. 1981a. Studies on the in vivo absorption of micellar solutions of tocopherol and tocopheryl acetate in the rat: Demonstration and partial characterization of a mucosal esterase localized to the endoplasmic reticulum of the enterocyte. J. Lipid Res. 22(5): 829–37. PMID 7288289. Retrieved January 16, 2009.
- Mathias, P. M., J. T. Harries, and D. P. Muller. 1981b. Optimization and validation of assays to estimate pancreatic esterase activity using well-characterized micellar solutions of cholesteryl oleate and tocopheryl acetate. J. Lipid Res. 22(1): 177–84. PMID 7217783. Retrieved January 16, 2009.
- McNeil, J. J., L. Robman, G. Tikellis, M. I. Sinclair, C. A. McCarty, and H. R. Taylor. 2004. Vitamin E supplementation and cataract: Randomized controlled trial. Ophthalmology 111(1): 75–84. PMID 14711717. Retrieved January 16, 2009.
- Miller, E., R. Pastor-Barriuso, D. Dalal, R. Riemersma, L. Appel, and E. Guallar. 2005. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142(1): 37–46. PMID 15537682. Retrieved January 16, 2009.
- Moriarty-Craige, S., J. Adkison, M. Lynn, G. Gensler, S. Bressler, D. Jones, and P. Sternberg. 2005. Antioxidant supplements prevent oxidation of cysteine/cystine redox in patients with age-related macular degeneration. Am J Ophthalmol 140(6): 1020–6. PMID 16376645. Retrieved January 16, 2009.
- National Cancer Institute (NCI). 2008a. The SELECT prostate cancer prevention trial. National Cancer Institute Oct. 27, 2008. Retrieved January 16, 2009.
- National Cancer Institute (NCI). 2008b Selenium and vitamin E cancer prevention trial (SELECT). National Cancer Institute Oct. 31, 2008. Retrieved January 16, 2009.
- Nursing in Practice (NIP). 2008. Vitamin E "linked to cancer." Nursing in Practice. Retrieved January 16, 2009.
- National Institutes of Health, Office of Dietary Supplements (NIH-ODS). n.d. Vitamin E fact sheet. National Institutes of Health. Retrieved January 16, 2009.
- O'Brien, L., and A. Pandit. 2006. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 1: CD003826. PMID 16437463. Retrieved January 16, 2009.
- Packer, L., S. U. Weber, and G. Rimbach. 2001. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J. Nutr. 131(2): 369S–73S. PMID 11160563. Retrieved January 17, 2009.
- Palmieri, B., G. Gozzi, and G. Palmieri. 1995. Vitamin E added silicone gel sheets for treatment of hypertrophic scars and keloids. Int. J. Dermatol. 34(7): 506–9. PMID 7591421. Retrieved January 17, 2009.
- Poston, L., A. Briley, P. Seed, F. Kelly, and A. Shennan. 2006. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): Randomised placebo-controlled trial. Lancet 367(9517): 1145–54. PMID 16616557. Retrieved January 16, 2009.
- Ricciarelli, R., F. Argellati, M. A. Pronzato, and C. Domenicotti. 2007. Vitamin E and neurodegenerative diseases. Mol. Aspects Med. 28(5-6): 591–606. PMID 17306357. Retrieved January 16, 2009.
- Rigotti, A. 2007. Absorption, transport, and tissue delivery of vitamin E. Mol. Aspects Med. 28(5-6): 423–36. PMID 17320165. Retrieved January 16, 2009.
- Rumbold, A., C. Crowther, R. Haslam, G. Dekker, and J. Robinson. 2006. Vitamins C and E and the risks of pre-eclampsia and perinatal complications. N Engl J Med 354(17): 1796–806. PMID 16641396. Retrieved January 16, 2009.
- Sen, C., S. Khanna, and S. Roy. 2006. Tocotrienols: Vitamin E beyond tocopherols. Life Sci 78(18): 2088–98. PMID 16458936.
- Sesso, H. D., et al. 2008. Vitamins E and C in the Prevention of Cardiovascular Disease in Men: The Physicians' Health Study II Randomized Controlled Trial. JAMA. 300(18): 2123-2133. Retrieved January 16, 2009.
- Shoulson, I. 1998. DATATOP: A decade of neuroprotective inquiry. Parkinson Study Group. Deprenyl And Tocopherol Antioxidative Therapy Of Parkinsonism. Ann. Neurol. 44(3, Suppl 1): S160–6. PMID 9749589. Retrieved January 16, 2009.
- Taylor, H., G. Tikellis, L. Robman, C. McCarty, and J. McNeil. 2002. Vitamin E supplementation and macular degeneration: randomised controlled trial. BMJ 325(7354): 11. PMID 12098721. Retrieved January 17, 2009.
- Traber, M. G., and J. Atkinson. 2007. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 43(1): 4–15. PMID 17561088. Retrieved January 17, 2009.
- Walker, M. 1992. New/old findings on unique vitamin E. Townsend Letter for Doctors and Patients 111: 826. Retrieved January 17, 2009.
- Wang, X., and P. Quinn. 1999. Vitamin E and its function in membranes. Prog Lipid Res 38(4): 309–36. PMID 10793887. Retrieved January 17, 2009.
- United States Department of Agriculture (USDA). 2008. Composition of foods raw, processed, prepared. USDA National Nutrient Database for Standard Reference, Release 20.
- van Leeuwen, R., S. Boekhoorn, J. Vingerling, J. Witteman, C. Klaver, A. Hofman, and P. de Jong. 2005. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA 294(24): 3101–7. PMID 16380590. Retrieved January 17, 2009.
- Vivekananthan, D., M. Penn, S. Sapp, A. Hsu, and E. Topol. 2003. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 361(9374): 2017–23. PMID 12814711. Retrieved January 17, 2009.
- Weiser, H., G. Riss, and A. W. Kormann. 1996. Biodiscrimination of the eight alpha-tocopherol stereoisomers results in preferential accumulation of the four 2R forms in tissues and plasma of rats. J. Nutr. 126(10): 2539–49. PMID 8857515. Retieved January 17, 2009.
- Zingg, J. M., and A. Azzi. 2004. Non-antioxidant activities of vitamin E. Curr. Med. Chem. 11(9): 1113–33.
External links
All links retrieved May 3, 2023.
- Vitamin E Medline Plus, Medical Encyclopedia, U.S. National Library of Medicine
- Jane Higdon, "Vitamin E", Micronutrient Information Center, Linus Pauling Institute
- Vitamin E (Tocopherols and Tocotrienols)
| Vitamins |
|---|
| All B vitamins | All D vitamins |
| Retinol (A) | Thiamine (B1) | Riboflavin (B2) | Niacin (B3) | Pantothenic acid (B5) | Pyridoxine (B6) | Biotin (B7) | Folic acid (B9) | Cyanocobalamin (B12) | Ascorbic acid (C) | Ergocalciferol (D2) | Cholecalciferol (D3) | Tocopherol (E) | Naphthoquinone (K) |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.