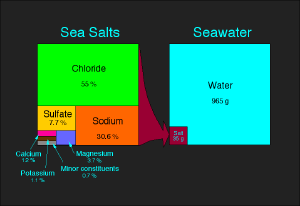
- For the chemical properties of salt, see sodium chloride; for the chemical term, see salt (chemistry).
- For other uses, see Salt (disambiguation).
Salt is a mineral, composed primarily of sodium chloride, which is commonly eaten by humans. There are different forms of salt: unrefined salt (such as sea salt), refined salt (table salt), and iodized salt. It is a crystalline solid, white, pale pink or light grey in color, normally obtained from sea water or rock deposits. Natural sea salt includes vital trace minerals in addition to the sodium chloride. Edible rock salts may be slightly greyish in color due to this mineral content.
Sodium and chlorine, the two components of salt, are necessary for the survival of all living creatures, including humans, but they need not be consumed as salt, where they are found together in very concentrated form. Some isolated cultures, such as the Yanomami in South America, have been found to consume little salt.[1] Salt is involved in regulating the water content (fluid balance) of the body. Salt flavor is one of the basic tastes. Salt cravings may be caused by trace mineral deficiencies as well as by a deficiency of sodium chloride itself.
Overconsumption of salt can increase the risk of health problems, including high blood pressure. In food preparation, salt is used as a preservative and as a seasoning.
Definition
A salt, in chemistry, is defined as the product formed from the neutralization reaction of acids and bases. Salts are ionic compounds composed of cations (positively charged ions) and anions (negative ions) so that the product is electrically neutral (without a net charge). These component ions can be inorganic such as chloride (Clβ), as well as organic such as acetate (CH3COOβ) and monoatomic ions such as fluoride (Fβ), as well as polyatomic ions such as sulfate (SO42β).
There are several varieties of salts. Salts that produce hydroxide ions when dissolved in water are basic salts and salts that produce hydronium ions in water acid salts. Neutral salts are those that are neither acid nor basic salts. Zwitterions contain an anionic center and a cationic center in the same molecule but are not considered to be salts. Examples include amino acids, many metabolites, peptides and proteins.
When salts are dissolved in water, they are called electrolytes, and are able to conduct electricity, a property that is shared with molten salts. Mixtures of many different ions in solutionβlike in the cytoplasm of cells, in blood, urine, plant saps and mineral watersβ usually do not form defined salts after evaporation of the water. Therefore, their salt content is given for the respective ions.
Appearance
Color
Salts can appear to be clear and transparent (sodium chloride), opaque, and even metallic and lustrous (iron disulfide). In many cases the apparent opacity or transparency are only related to the difference in size of the individual monocrystals. Since light reflects from the phase boundaries, larger crystals tend to be transparent, while poly-crystalline aggregates look like white powders. Of course, some salts are inherently opaque.
Salts exist in the full range of different colors. Examples are: yellow (sodium chromate), orange (potassium dichromate), red (mercury sulfide), mauve (cobalt chloride hexahydrate), blue (copper sulfate pentahydrate, ferric hexacyanoferrate), green (nickel oxide), colorless (magnesium sulfate), white, and black (manganese dioxide). Most minerals and inorganic pigments as well as many synthetic organic dyes are salts.
Taste
Different salts can elicit all five basic tastes, e.g., salty (sodium chloride), sweet (lead diacetate; but which will cause lead poisoning if ingested), sour (potassium bitartrate), bitter (magnesium sulfate), and umami or savory (monosodium glutamate).
Odor
Salts of strong acids and strong bases ("strong salts") are non-volatile and odorless, while salts of either weak acids or weak bases ("weak salts") may smell after the conjugate acid (e.g. acetates like acetic acid (vinegar) and cyanides like hydrogen cyanide (almonds) or the conjugate base (e.g. ammonium salts like ammonia) of the component ions. That slow, partial decomposition is usually accelerated by presence of water, since hydrolysis is the other half of the reversible reaction equation of formation of weak salts.
Nomenclature
The name of a salt starts with the name of the cation (e.g. sodium or ammonium) followed by the name of the anion (e.g., chloride or acetate). Salts are often referred to only by the name of the cation (e.g., sodium salt or ammonium salt) or by the name of the anion (e.g., chloride or acetate).
Formation
Salts are formed by a chemical reaction between:
- A base and an acid anhydride, e.g. 2 NaOH + Cl2O β 2 NaClO + H2O
Salts can also form if solutions of different salts are mixed, their ions recombine, and the new salt is insoluble and precipitates (see: solubility equilibrium).
Salt-forming ions
Common salt-forming cations include:
- ammonium NH4+
- calcium Ca2+
- iron Fe2+ and Fe 3+
- magnesium Mg2+
- potassium K+
- pyridinium C5H5NH+
- quaternary ammonium NR4+
- sodium Na+
Common salt-forming anions (and the name of the parent acids in parentheses) include:
- acetate CH3COOβ (acetic acid)
- carbonate CO32β (carbonic acid)
- chloride Clβ (hydrochloric acid)
- citrate HOC(COOβ)(CH2COOβ)2 (citric acid)
- cyanide Cβ‘Nβ (hydrogen cyanide)
- hydroxide OHβ (water)
- nitrate NO3β (nitric acid)
- nitrite NO2β (nitrous acid)
- oxide O2β (water)
- phosphate PO43β (phosphoric acid)
- sulfate SO42β (sulfuric acid)
History of the use of common salt
Salt's preservative ability was a foundation of civilization. It eliminated dependency on the seasonal availability of food, allowed travel over long distances, and was a vital food additive. However, because salt (NaCl) was difficult to obtain, it became a highly valued trade item throughout history. Until the 1900s, salt was one of the prime movers of national economies and wars. Salt was often taxed; research has discovered this practice to have existed as early as the twentieth century B.C.E. in China.
The first registers of salt use were produced around 4000 B.C.E. in Egypt, and later in Greece and Rome. Salt was very valuable and used to preserve and flavor foods. In Ancient Rome, salt was used as a currency. The Latin word salarium meaning a payment made in salt, is the root of the word "salary." Unfortunately for those paid with salt, it was easily ruined by rain and other weather conditions. Payments to Roman workers and soldiers were made in salt.[2]
From the Phoenicians dates the evidence of harvesting solid salt from the sea. They also exported it to other civilizations. As a result of the increased salt supply from the sea, the value of salt depreciated. The harvest method used was flooding plains of land with seawater, then leaving the plains to dry. After the water dried, the salt which was left was collected and sold.
In the Mali Empire, merchants in twelfth century Timbuktuβthe gateway to the Sahara Desert and the seat of scholarsβvalued salt (NaCl) enough to buy it for its weight in gold; this trade led to the legends of the incredibly wealthy city of Timbuktu, and fueled inflation in Europe, which was importing the salt.[3]
During his protests in India, Mohandas Gandhi performed the famous salt march to challenge the British-imposed monopoly on salt.
In religion
Among the ancients, as with ourselves, "sol" (sun) and "sal" (salt) were considered essential to the maintenance of life.
There are 35 references (verses) to salt in the Bible (King James Version), the most familiar probably being the story of Lot's wife, who was turned into a pillar of salt when she disobeyed the angels and looked back at the wicked city of Sodom (Genesis 19:26). In the Sermon on the Mount, Jesus also referred to his followers as the "salt of the earth." The apostle Paul also encouraged Christians to "let your conversation be always full of grace, seasoned with salt" (Colossians 4:6) so that when others enquire about their beliefs, the Christian's answer generates a 'thirst' to know more about Christ. Salt is mandatory in the rite of the Tridentine Mass. Salt is used in the third item (which includes an Exorcism) of the Celtic Consecration (refer Gallican rite) that is employed in the Consecration of a Church. Salt may be added to the water "where it is customary" in the Roman Catholic rite of Holy water. The earliest Biblical mention of salt appears to be in reference to the destruction of Sodom and Gomorrah (Genesis xix. 24-26) When King Abimelech destroyed the city of Shechem, held to have occurred in the thirteenth century B.C.E., he is said to have "sowed salt on it," this phrase expressing the completeness of its ruin. (Judges ix. 45.)
In the native Japanese religion Shinto, salt is used for ritual purification of locations and people, such as in Sumo Wrestling.
In Aztec mythology, Huixtocihuatl was a fertility goddess who presided over salt and salt water.
Forms of salt
Unrefined salt
Different natural salts have different mineralities, giving each one a unique flavor. Fleur de sel, natural sea salt harvested by hand, has a unique flavor varying from region to region.
Some assert that unrefined sea salt is more healthy than refined salts.[4] However, completely raw sea salt is bitter due to magnesium and calcium compounds, and thus is rarely eaten. Other people think that raw sea and rock salts do not contain sufficient iodine salts to prevent iodine deficiency diseases like hypothyroidism.[5]
Refined salt
Refined salt, which is most widely used presently, is mainly sodium chloride. Food grade salt accounts for only a small part of salt production in industrialised countries (3% in Europe[6]) although world-wide, food uses account for 17.5% of salt production[7]. The majority is sold for industrial use, from manufacturing pulp and paper to setting dyes in textiles and fabric, to producing soaps and detergents, and has great commercial value.

The manufacture and use of salt is one of the oldest chemical industries.[8] Salt is also obtained by evaporation of sea water, usually in shallow basins warmed by sunlight;[9] salt so obtained was formerly called bay salt, and is now often called sea salt or solar salt. Refined salt is also prepared from rock salt: mineral deposits high in salt. These rock salt deposits were formed by the evaporation of ancient salt lakes.[10] These deposits may be mined conventionally or through the injection of water. Injected water dissolves the salt, and the brine solution can be pumped to the surface where the salt is collected.
After the raw salt is obtained, it is refined to purify it and improve its storage and handling characteristics. Purification usually involves recrystallization. In recrystallization, a brine solution is treated with chemicals that precipitate most impurities (largely magnesium and calcium salts).[11] Multiple stages of evaporation are then used to collect pure sodium chloride crystals, which are kiln-dried.
Since the 1950s it has been common to add a trace of sodium hexacyanoferrate II to the brine, this acts as an anticaking agent by promoting irregular crystals.[12] Other anticaking agents (and potassium iodide, for iodized salt) may be added after crystallization. These agents are hygroscopic chemicals which absorb humidity, keeping the salt crystals from sticking together. Some anticaking agents used are tricalcium phosphate, calcium or magnesium carbonates, fatty acid salts (acid salts), magnesium oxide, silicon dioxide, calcium silicate, sodium alumino-silicate, and alumino-calcium silicate. Concerns have been raised regarding the possible toxic effects of aluminium in the latter two compounds, however both the European Union and the United States Food and Drug Administration (FDA) permit their use.[13] The refined salt is then ready for packing and distribution.
Table salt
Table salt is refined salt, 99 percent sodium chloride.[14][15] It usually contains substances that make it free flowing (anticaking agents) such as sodium silicoaluminate or magnesium carbonate. It is common practice to put a few grains of uncooked rice in salt shakers to absorb extra moisture when anticaking agents are not enough.
Iodized salt
Iodized salt (BrE: iodised salt), table salt mixed with a minute amount of sodium iodide, iodate, or sometimes potassium iodide, is used to help reduce the chance of iodine deficiency in humans. Iodine deficiency commonly leads to thyroid gland problems, specifically endemic goiter. Endemic goiter is a disease characterized by a swelling of the thyroid gland, usually resulting in a bulbous protrusion on the neck. While only tiny quantities of iodine are required in a diet to prevent goiter, the United States Food and Drug Administration recommends (21 CFR 101.9 (c)(8)(iv)) 150 microgrammes of iodine per day for both men and women, and there are many places around the world where natural levels of iodine in the soil are low and the iodine is not taken up by vegetables.
Today, iodized salt is more common in the United States, Australia and New Zealand than in Britain. Table salt is also often iodizedβa small amount of potassium iodide (in the US) or potassium iodate (in the EU) is added as an important dietary supplement. Table salt is mainly employed in cooking and as a table condiment. Iodized table salt has significantly reduced disorders of iodine deficiency in countries where it is used.[16] Iodine is important to prevent the insufficient production of thyroid hormones (hypothyroidism), which can cause goitre, cretinism in children, and myxedema in adults.
Fluorinated salt
In some European countries where fluoridation of drinking water is not practiced, fluorinated table salt is available. In France, 35 percent of sold table salt contains sodium or potassium fluoride.[17] Another additive, especially important for pregnant women is Folic acid (B vitamin) giving the table salt a yellow color.
Salty condiments
In many Asian cultures, table salt is not traditionally used as a condiment.[18]However, condiments such as soy sauce, fish sauce and oyster sauce tend to have a high salt content and fill much the same role as a salt-providing table condiment that table salt serves in western cultures.
Health effects of salt intake
Sodium is one of the primary electrolytes in the body. All three electrolytes (sodium, potassium, and calcium) are available in unrefined salt, as are other vital minerals needed for optimal bodily function. Too much or too little salt in the diet can lead to muscle cramps, dizziness, or even an electrolyte disturbance, which can cause severe, even fatal, neurological problems.[19] Drinking too much water, with insufficient salt intake, puts a person at risk of water intoxication. Salt is even sometimes used as a health aid, such as in treatment of dysautonomia.[20]
People's risk for disease due to salt intake that is too low or too high varies, due to biochemical individuality. Some have asserted that while the risks of consuming too much salt are real, the risks have been exaggerated for most people, or that the studies done on the consumption of salt can be interpreted in many different ways.[21] [22]
Excess salt consumption has been linked to:
- exercise-induced asthma.[23] On the other hand, another source counters, "β¦we still don't know whether salt contributes to asthma. If there is a link then it's very weakβ¦".[24]
- heartburn[25].
- osteoporosis: One report shows that a high salt diet does reduce bone density in girls.[26]. Yet "While high salt intakes have been associated with detrimental effects on bone health, there are insufficient data to draw firm conclusions." ([27], p3)
- Gastric cancer (Stomach cancer) is associated with high levels of sodium, "but the evidence does not generally relate to foods typically consumed in the UK." ([28]) However, in Japan, salt consumption is higher.[29]
- hypertension (high blood pressure): "Since 1994, the evidence of an association between dietary salt intakes and blood pressure has increased. The data have been consistent in various study populations and across the age range in adults." ([30]). "The CMO [Chief Medical Officer] of England, in his Annual Report (DH, 2001), highlighted that people with high blood pressure are three times more likely to develop heart disease and stroke, and twice as likely to die from these diseases than those with normal levels."([31]). One study found that low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men [32].
- left ventricular hypertrophy (cardiac enlargement): "Evidence suggests that high salt intake causes left ventricular hypertrophy, a strong risk factor for cardiovascular disease, independently of blood pressure effects." ([33]) "β¦ there is accumulating evidence that high salt intake predicts left ventricular hypertrophy." ([34]) Excessive salt (sodium) intake, combined with an inadequate intake of water, can cause hypernatremia. It can exacerbate renal disease.[35]
- edema (BE: oedema): A decrease in salt intake has been suggested to treat edema (BE: oedema) (fluid retention).[36]
- duodenal ulcers and gastric ulcers [37]
A large scale study by Nancy Cook, et al. shows that people with high-normal[38] blood pressure who significantly reduced the amount of salt in their diet decreased their chances of developing cardiovascular disease by 25 percent over the following 10 to 15 years. Their risk of dying from cardiovascular disease decreased by 20 percent.[39][40]
Recommended intake
This section summarizes the salt intake recommended by the health agencies of various countries. Recommendations tend to be similar. Note that targets for the population as a whole tend to be pragmatic (what is achievable) while advice for an individual is ideal (what is best for health). For example, in the UK target for the population is "eat no more than 6 g a day" but for a person is 4 g.
Intakes can be expressed variously as salt or sodium and in various units.
- 1 g sodium = 1,000 mg sodium = 42 mmol sodium = 2.5 g salt
United Kingdom: In 2003, the UK's Scientific Advisory Committee on Nutrition (SACN) recommended that, for a typical adult, the Reference Nutrient Intake is 4 g salt per day (1.6 g or 70 mmol sodium). However, average adult intake is two and a half times the Reference Nutrient Intake for sodium. "Although accurate data are not available for children, conservative estimates indicate that, on a body weight basis, the average salt intake of children is higher than that of adults." SACN aimed for an achievable target reduction in average intake of salt to 6 g per day (2.4 g or 100 mmol sodium) β this is roughly equivalent to a teaspoonful of salt. The SACN recommendations for children are:
- 0β6 months old: less than 1 g/day
- 7β12 months: 1 g/day
- 1β3 years: 2 g/day
- 4β6 years: 3 g/day
- 7β10 years: 5 g/day
- 11β14 years: 6 g/day
SACN states, "The target salt intakes set for adults and children do not represent ideal or optimum consumption levels, but achievable population goals."[41]
Republic of Ireland: The Food Safety Authority of Ireland endorses the UK targets "emphasising that the RDA of 1.6 g sodium (4 g salt) per day should form the basis of advice targeted at individuals as distinct from the population health target of a mean salt intake of 6 g per day."([42])
Canada: Health Canada recommends an Adequate Intake (AI) and an Upper Limit (UL) in terms of sodium.
- 0β6 months old: 0.12 g/day (AI)
- 7β12 months: 0.37 g/day (AI)
- 1β3 years: 1 g/day (AI) 1.5 g/day (UL)
- 4β8 years: 1.2/day (AI) 1.9 g/day (UL)
- 9β13 years: 1.5 g/day (AI) 2.2 g/day (UL)
- 14β50 years: 1.5 g/day (AI) 2.3 g/day (UL)
- 51β70 years: 1.3 g/day (AI) 2.3 g/day (UL)
- 70 years and older: 1.2 g/day (AI) 2.3 g/day (UL)[43]
- Adequate Intake (AI) 0.46 β 0.92 g sodium = 1.2 β 2.3g salt
- Upper Limit (UL)) 2.3 g sodium = 5.8 g salt
Australia: The recommended dietary intake (RDI) is 0.92 gβ2.3 g sodium per day (= 2.3 gβ5.8 g salt)[44]
USA: The Food and Drug Administration itself does not make a recommendation[45] but refers readers to Dietary Guidelines for Americans 2005. These suggest that US citizens should consume less than 2,300 mg of sodium (= 2.3 g sodium = 5.8 g salt) per day. [46]
Labeling
UK: The Food Standards Agency defines the level of salt in foods as follows: "High is more than 1.5g salt per 100g (or 0.6g sodium). Low is 0.3g salt or less per 100g (or 0.1g sodium). If the amount of salt per 100g is in between these figures, then that is a medium level of salt." In the UK, foods produced by some supermarkets and manufacturers have βtraffic lightβ colors on the front of the pack: Red (High), Amber (Medium), or Green (Low).[47]
USA: The FDA Food Labeling Guide stipulates whether a food can be labelled as "free," "low," or "reduced/less" in respect of sodium. When other health claims are made about a food (e.g., low in fat, calories, etc.), a disclosure statement is required if the food exceeds 480mg of sodium per 'serving.'[48]
Campaigns
In 2004, Britain's Food Standards Agency started a public health campaign called "Salt - Watch it," which recommends no more than 6g of salt per day; it features a character called Sid the Slug and was criticised by the Salt Manufacturers Association (SMA).[49] The Advertising Standards Authority did not uphold the SMA complaint in its adjudication.[50].
The Menzies Research Institute in Tasmania, Australia, maintains a website [51] dedicated to educating people about the potential problems of a salt-laden diet.
Salt substitutes
Salt intake can be reduced by simply reducing the quantity of salty foods in a diet, without recourse to salt substitutes. Salt substitutes have a taste similar to table salt and contain mostly potassium chloride, which will increase potassium intake. Excess potassium intake can cause hyperkalemia. Various diseases and medications may decrease the body's excretion of potassium, thereby increasing the risk of hyperkalemia. If you have kidney failure, heart failure or diabetes, seek medical advice before using a salt substitute. A manufacturer, LoSalt, has issued an advisory statement[52] that people taking the following prescription drugs should not use a salt substitute: Amiloride, Triamterene, Dytac, Spironolactone, Aldactone, Eplerenone and Inspra.
Production trends
Salt is produced by evaporation of seawater or brine from other sources, such as brine wells and salt lakes, and by mining rock salt, called halite. In 2002, total world production was estimated at 210 million metric metric tons, the top five producers being the United States (40.3 million metric tons), China (32.9), Germany (17.7), India (14.5), and Canada (12.3).[53] Note that these figures are not just for table salt but for sodium chloride in general.
See also
- Electrolyte
- Halide
- Sodium chloride
- Sea salt
- History of salt
- Fleur de sel
- Curing (food preservation)
- Acid salt
- Alkali salts
- Edible salt
- Ionic bonds
- Kosher salt
- Natron
- Old Salt Route
- Road salt
- Salting the earth is the deliberate massive use of salt to render a soil unsuitable for cultivation and thus discourage habitation.
- Sodium
- Table salt
- Zwitterion
- Salinity
- hypertension
Notes
- β Yanomami Indians in the INTERSALT study. Retrieved July 28, 2007.
- β David Bloch, Economics of NaCl: Salt made the world go round. www.salt.org. Retrieved July 28, 2007.
- β Janice McDonald.Take a journey to Timbuktu. CNN News.Retrieved July 28, 2007.
- β Susan Dearing. Sea Salt is good for you and is made in Colima! Retrieved July 28, 2007.
- β Iodine in non-iodized sea salt. Salt Institute.
- β Salt and its Applications. European Salt Producers' Association. Retrieved July 28, 2007.
- β Asian salt production increased rapidly since 2003. Roskill Information Services. Retrieved July 28, 2007.
- β Archeology Salt.org. Retrieved July 28, 2007.
- β ACTIVITY THREE - THE WATER CYCLE Nauticus - Weather Curriculum. Retrieved July 28, 2007.
- β What is Salt?. UK Salt Manufacturers' Association. Retrieved July 28, 2007.
- β About Salt: Production. The Salt Manufacturers Association. Retrieved July 30, 2007.
- β Salt and the Chemical Revolution: 8 Salt Making in the 20th century. The Salt Manufacturers Association. Retrieved July 30, 2007.
- β Daniels Burgess, Wilella Daniels and Mason, April C. What Are All Those Chemicals in My Food? Purdue University, Cooperative Extension Service. Retrieved July 30, 2007.
- β Nutritional analysis provided with Tesco Table Salt, from Tesco Stores Ltd (UK) states 38.9 percent sodium by weight which equals 98.9 percent sodium chloride
- β Salt Products - Table Salt. WA Salt Supply. Retrieved July 30, 2007.
- β Iodized Salt Salt Institute.
- β [1] Assessment of the health risks from noncompliance with drinking water parametric values. AFSSA. Retrieved July 30, 2007.
- β Matthew Amster-Burton. 2001. The Salt of Southeast Asia. The Seattle Times. Retrieved July 30, 2007.
- β Salt. Australia: Better Health Channel (Australia, Victoria). Retrieved July 30, 2007.
- β Dysautonomia. Cleveland Clinic Health Information Center.
- β Salt and other wounds. Why Files article. Retrieved July 30, 2007.
- β Gary Taubes. 1998. The (Political) Science of Salt, Science 281 (August 14, 1998) (5379): 898 - 907. Retrieved July 30, 2007.
- β Tracy James. 2003. Exercise-induced asthma more clearly linked to high-salt diet. IU Home Pages. Retrieved July 30, 2007.
- β Dr. Trisha Macnair, "Does eating salt make asthma worse?" BBC Health.
- β Study adds salt to suspected triggers for heartburn Everybody.
- β High salt diet reduces bone density in girls. Food Navigator. Retrieved July 30, 2007.
- β Salt and Health. Scientific Advisory Committee on Nutrition (SACN). Retrieved July 30, 2007.
- β Ibid., 18
- β Salt raises 'stomach cancer risk'. BBC News. Retrieved July 30, 2007.
- β SACN, 3
- β Ibid., 14
- β Michael H. Alderman, Shantha Madhavan, Hillel Cohen, Jean E. Sealey, and John H. Laragh, 1992. Low Urinary Sodium Is Associated With Greater Risk of Myocardial Infarction Among Treated Hypertensive Men Hypertension 25(1995): 1144-1152. Retrieved July 30, 2007.
- β SACN 3
- β Salt and Health: Review of the Scientific Evidence and Recommendations for Public Policy in Ireland Food Safety Authority of Ireland. Retrieved July 30, 2007.
- β oz
- β Fluid retention Australia: Better Health Channel (Australia, Victoria). Retrieved July 30, 2007.
- β High-salt diet link to ulcer risk. BBC. Retrieved July 30, 2007.
- β [http://www.vaughns-1-pagers.com/medicine/blood-pressure.htm Blood Pressure Chart ]. Vaughns. Retrieved July 30, 2007.
- β Cutting salt 'reduces heart risk'. BBC News. Retrieved July 30, 2007.
- β Eating less salt could prevent cardiovascular disease Eurikalert. Retrieved July 30, 2007.
- β SACN
- β fsai, 16
- β Dietary Reference Intakes (look for Sodium) Health Canada. Retrieved August 2, 2007.
- β Salt Better Health Channel (Australia, Victoria). Retrieved August 2, 2007.
- β A Pinch of Controversy Shakes Up Dietary Salt U.S. Food and Drug Administration. Retrieved August 2, 2007.
- β "Sodium and Potassium" Department of Health and Human Services (HHS) and the Department of Agriculture (USDA). Retrieved August 2, 2007.
- β Understanding labels Food Standards Agency. Retrieved August 2, 2007.
- β A Food Labeling GuideβAppendix A Food and Drug Administration. Retrieved August 2, 2007.
- β New salt campaign under attack Salt Manufacturers Association press release. Retrieved August 2, 2007.
- β Broadcast Advertising Adjudications: 20 April 2005 Advertising Standards Authority.
- β Salt Matters Menzies Research Institute. Retrieved August 2, 2007.
- β Advisory Statement LoSalt. Retrieved August 2, 2007.
- β Susan R. Feldman. Sodium chloride. John Wiley & Sons, Inc. Published online 2005. Sodium Chloride Kirk-Othmer Encyclopedia of Chemical Technology. Retrieved August 2, 2007.
ReferencesISBN links support NWE through referral fees
- Kurlansky, Mark. 2002. Salt: A World History. New York, NY: Walker Publishing Company. ISBN 0142001619.
- __________. and S. D. Schindler. 2006. The Story of Salt. New York, NY: G.P. Putnam's Sons. ISBN 0399239987 β a children's book about salt.
- Laszlo, Pierre. 2002. Salt: Grain of Life. Arts and traditions of the table. New York, NY: Columbia University Press, 2001. ISBN 0060084685.
- UK: Department of Health, Dietary Reference Values for Food Energy and Nutrients for the UK: Report of the Panel on DRVs of the Committee on the Medical Aspects of Food Policy. The Stationery Office.
External links
All links retrieved December 22, 2022.
- Cook's Thesaurus: Salt (Summary and descriptions of edible salts).
- "Effect of longer-term modest salt reduction on blood pressure" The Cochrane Collaboration.
- Salt: The Forgotten Killer CSPI page.
- Salt matters - talk by Dr Trevor Beard, Menzies Research Institute (ABC Radio National 4 February 2007). Ockham's Razor.
- David Bloch. Economics of NaCl: Salt made the world go round.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.